The primary function of a three-chamber H-type electrolytic cell is to create physically separate but ionically connected environments for conducting complex, multi-step electrochemical reactions. Unlike simpler cells, this design allows researchers to generate a reactive chemical species in one chamber, isolate it, and then have it react or be transformed in a second chamber, all while the corresponding counter-reaction occurs undisturbed in a third.
The core advantage of the three-chamber design is its ability to manage unstable chemical intermediates. It provides a dedicated space for these intermediates to exist and react without being immediately consumed or destroyed by the starting materials or the opposing electrode.
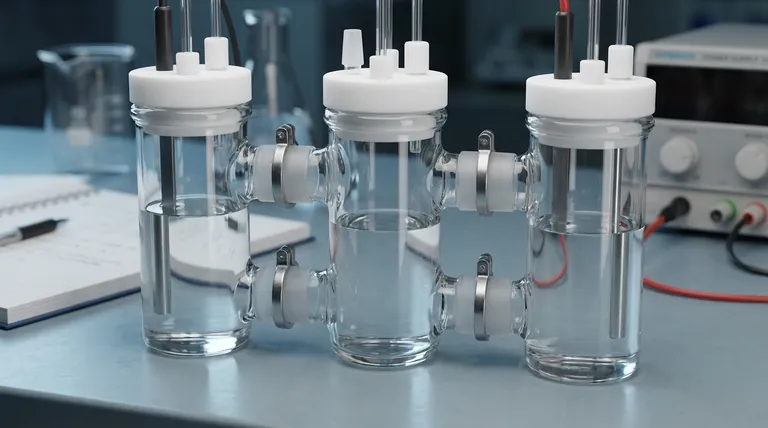
The Foundation: What Is an Electrolytic Cell?
Driving Non-Spontaneous Reactions
An electrolytic cell is a device that uses external electrical energy, typically from a power supply, to force a chemical reaction that would not happen on its own.
The Core Process: Electrolysis
This process is called electrolysis, where electricity is used to decompose chemical compounds. A classic example is using an electrolytic cell to split water into its constituent parts: hydrogen and oxygen gas.
Basic Components
At its heart, any electrolytic cell consists of two electrodes—an anode (positive) and a cathode (negative)—immersed in a conductive liquid or solution called an electrolyte.
The Problem with Simpler Cells
Unwanted Side Reactions
In a simple, single-chamber cell, the products generated at the anode can drift over to the cathode (and vice versa). This "crossover" can lead to unwanted side reactions, reducing the efficiency and purity of the desired product.
The Standard H-Type Cell Solution
A standard two-chamber H-type cell solves this primary problem. It uses an ion-exchange membrane to separate the anode and cathode compartments, physically blocking products from crossing over while still allowing ions to pass through and complete the electrical circuit.
A Lingering Limitation
However, even a two-chamber cell is limited. It is designed for a single, direct redox reaction. It cannot effectively manage a process where an unstable intermediate product is formed, as that intermediate could still react with the starting material or be destroyed at its own electrode.
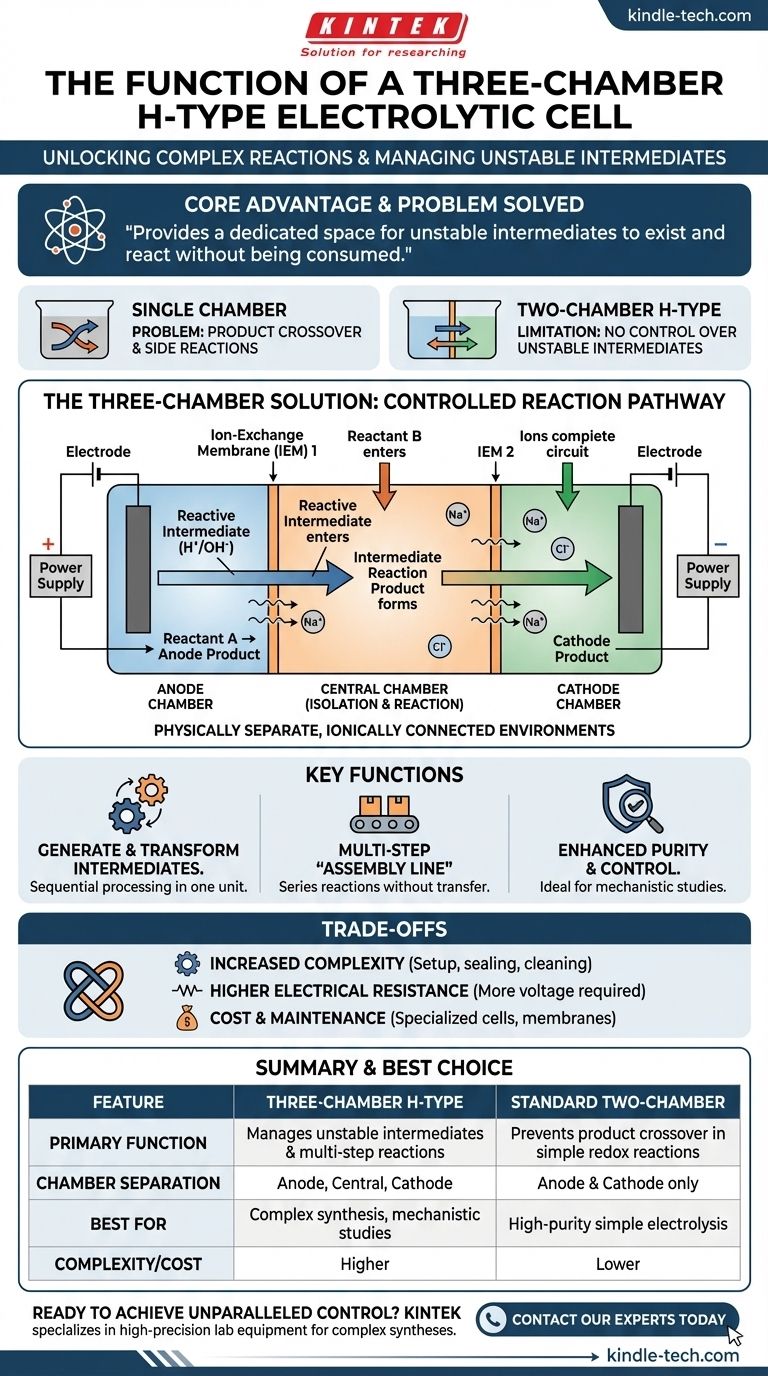
The Three-Chamber Solution: Unlocking Complex Reactions
Introducing the Third Chamber
The three-chamber H-type cell adds a central compartment, separating the anode and cathode chambers. This middle chamber is connected to the outer two by ion-exchange membranes, creating a highly controlled reaction pathway.
Key Function 1: Generating and Transforming Intermediates
This is the most critical function. It allows for a sequential process within a single piece of equipment.
For example, a researcher can:
- Generate a reactive intermediate at the anode in Chamber 1.
- Have that intermediate migrate into the central Chamber 2.
- Introduce another substance into Chamber 2 to react specifically with that intermediate.
- All while the cathode reaction proceeds independently in Chamber 3.
This setup prevents the unstable intermediate from ever reaching the cathode, where it would be instantly destroyed.
Key Function 2: Multi-Step "Assembly Line" Reactions
This design essentially creates an electrochemical assembly line. You can perform distinct but related electrolytic steps in series without having to isolate, purify, and transfer products between separate pieces of equipment.
Key Function 3: Enhanced Purity and Control
By completely isolating the initial reactants, the intermediate species, and the final products from one another, the three-chamber cell offers unparalleled control. This leads to much higher purity and a more precise understanding of the reaction mechanism.
Understanding the Trade-offs
Increased Complexity
The sophisticated design is more difficult to set up, requires careful sealing, and can be more challenging to clean and troubleshoot than simpler cells.
Higher Electrical Resistance
Each additional membrane and compartment adds to the overall internal resistance of the cell. This means more voltage (and thus, more energy) is required to drive the desired reaction at a given speed.
Cost and Maintenance
These specialized cells are more expensive. The ion-exchange membranes also require careful handling and occasional replacement, adding to the operational cost and maintenance burden.
Making the Right Choice for Your Goal
Ultimately, the choice of cell depends entirely on the complexity of the chemical reaction you need to perform.
- If your primary focus is simple electrolysis (e.g., water splitting): A basic single-compartment cell is often sufficient and most energy-efficient.
- If your primary focus is preventing product crossover to achieve high purity: A standard two-chamber H-type cell is the ideal choice.
- If your primary focus is synthesizing or studying a reactive intermediate in a multi-step process: The three-chamber H-type cell is specifically designed for this task and is often the only viable option.
Selecting the correct electrolytic cell is about matching the tool's complexity to the complexity of the chemical transformation you aim to achieve.
Summary Table:
| Feature | Three-Chamber H-Type Cell | Standard Two-Chamber Cell |
|---|---|---|
| Primary Function | Manages unstable intermediates & multi-step reactions | Prevents product crossover in simple redox reactions |
| Chamber Separation | Anode, Central (for intermediates), Cathode | Anode and Cathode only |
| Best For | Complex synthesis, mechanistic studies | High-purity simple electrolysis |
| Complexity/Cost | Higher setup and maintenance | Lower setup and maintenance |
Ready to achieve unparalleled control in your electrochemical research?
KINTEK specializes in high-precision lab equipment, including advanced electrolytic cells designed for complex syntheses and mechanistic studies. Our three-chamber H-type cells provide the dedicated environments you need to generate, isolate, and transform reactive intermediates with confidence.
Contact our experts today to discuss how our solutions can enhance your laboratory's capabilities and accelerate your research. Get in touch with KINTEK now!
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results
- What experimental conditions need to be controlled when using an H-type electrolytic cell? Ensure Reliable and Repeatable Results
- How should failures or malfunctions of an H-type electrolytic cell be handled? A Guide to Safe and Effective Troubleshooting
- What are the proper storage conditions for an H-type electrolytic cell? Ensure Long-Term Reliability and Accurate Results
- What is the function of an H-type exchangeable membrane electrolytic cell? Master Precise Reaction Control



















