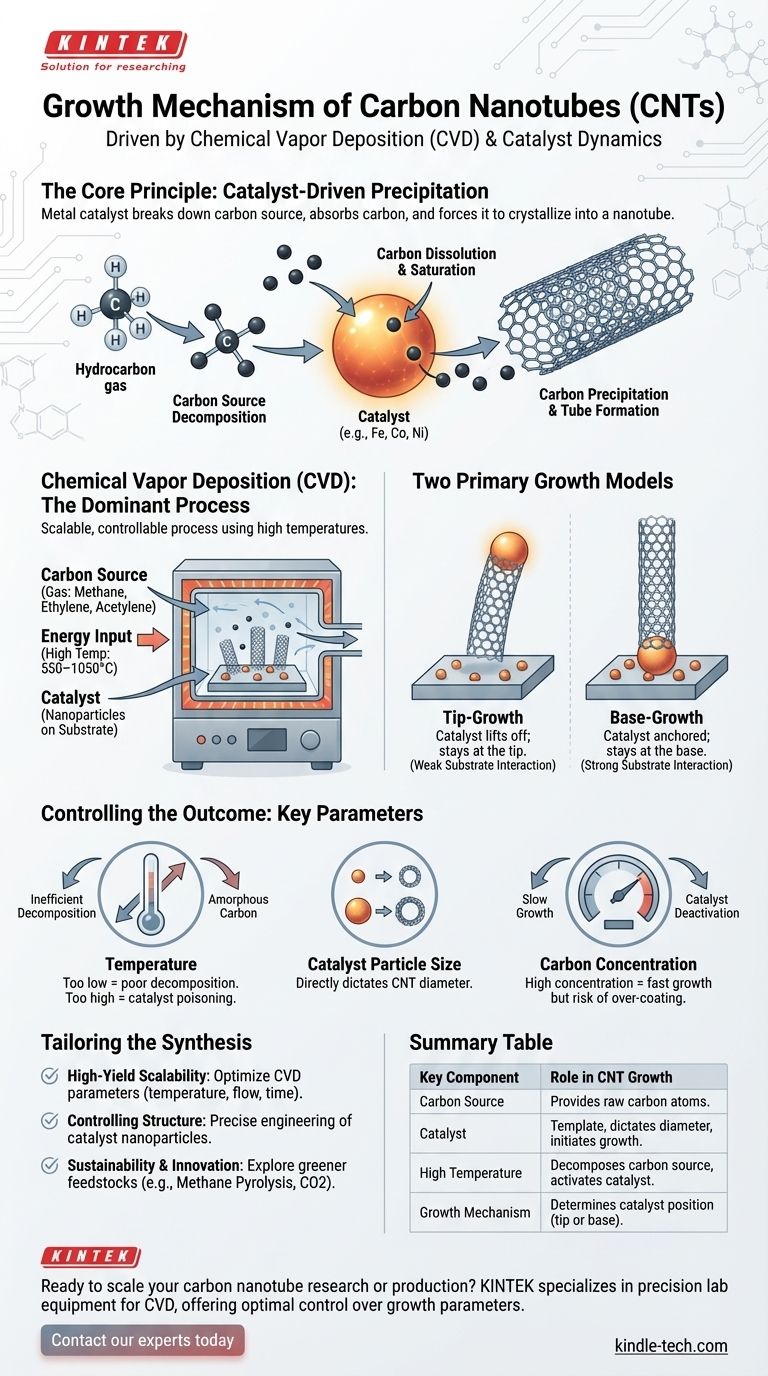
In short, carbon nanotubes grow when a carbon-containing gas decomposes on the surface of a tiny metal catalyst particle at high temperatures. The carbon atoms dissolve into the metal, and when it becomes saturated, they precipitate out in a self-assembling cylindrical structure, forming the nanotube. This process is predominantly achieved through a method called Chemical Vapor Deposition (CVD).
The core principle of nanotube growth is catalyst-driven precipitation. Think of the metal catalyst as a microscopic template or seed; it breaks down a carbon source, absorbs the carbon, and then forces the carbon to crystallize and grow outwards as a perfect hexagonal tube.

The Central Role of Chemical Vapor Deposition (CVD)
While older methods like laser ablation exist, Chemical Vapor Deposition (CVD) is the dominant commercial process for producing carbon nanotubes (CNTs) today. Its popularity stems from its scalability and the high degree of control it offers over the final product.
The Three Essential Ingredients
The CVD process for CNT growth fundamentally relies on a precise combination of three components in a high-temperature furnace.
- A Carbon Source: This is typically a hydrocarbon gas like methane, ethylene, or acetylene. The bonds in this gas are broken to provide the raw carbon atoms for building the nanotube.
- Energy Input: High temperatures (often 550–1050°C) are required. This energy serves to break down the carbon source gas and keep the catalyst particle in an active, quasi-liquid state.
- A Catalyst: This is the most critical component. Nanoparticles of metals like iron, cobalt, or nickel are deposited onto a substrate. These particles act as the sites where the entire growth process is initiated and sustained.
Unpacking the Growth Mechanism: Tip vs. Base Growth
Once the ingredients are in place, the growth occurs in a series of predictable steps. The specific way the nanotube forms is described by two primary models: "tip-growth" and "base-growth."
Step 1: Carbon Source Decomposition
The high temperature causes the hydrocarbon gas to decompose, or "crack," on the surface of the metal catalyst particle, releasing free carbon atoms.
Step 2: Carbon Dissolution and Saturation
These carbon atoms then dissolve into the metal particle. This process continues until the catalyst particle becomes supersaturated with carbon, much like sugar dissolving in water until no more can be absorbed.
Step 3: Carbon Precipitation and Tube Formation
Once supersaturated, the catalyst expels the carbon. The carbon atoms precipitate out of the particle and self-assemble into the stable, hexagonal lattice structure of a graphene sheet, which then closes into a tube.
The "Tip-Growth" Model
In this model, the interaction between the catalyst particle and the support substrate is weak. As the nanotube forms, it lifts the catalyst particle off the substrate. The result is a nanotube with the catalyst particle located at its growing tip.
The "Base-Growth" Model
Conversely, if the interaction between the catalyst and the substrate is strong, the particle remains anchored. The carbon precipitates from the top of the catalyst, and the nanotube grows upwards, leaving the catalyst at its base.
Understanding the Trade-offs and Controlling the Outcome
Mastering the growth mechanism is about manipulating key parameters to control the outcome. The productivity and quality of the final CNTs are directly tied to how well these variables are managed.
The Influence of Temperature
Temperature is a critical operating parameter. If it's too low, the carbon source won't decompose efficiently. If it's too high, you risk forming undesirable amorphous carbon instead of structured nanotubes, which can poison the catalyst.
The Role of the Catalyst Particle
The size of the catalyst nanoparticle directly dictates the diameter of the carbon nanotube. This is one of the most powerful control levers in synthesis. A smaller particle produces a smaller-diameter tube.
The Impact of Carbon Concentration
The concentration of the carbon source gas must be carefully balanced. A high concentration can increase the growth rate, but it also increases the risk of catalyst deactivation due to an over-coating of amorphous carbon.
Making the Right Choice for Your Goal
Understanding the fundamentals of the growth mechanism allows you to tailor the synthesis process to your specific objective.
- If your primary focus is high-yield scalability: Concentrate on optimizing the process parameters of CVD (temperature, gas flow, residence time) to maximize productivity and efficiency.
- If your primary focus is controlling nanotube structure (e.g., diameter or single vs. multi-wall): Your effort should be on the precise engineering of the catalyst nanoparticles, as they act as the template for growth.
- If your primary focus is sustainability and innovation: Explore emerging methods like methane pyrolysis or using captured CO2 as a feedstock, which represent the future of greener CNT production.
Ultimately, controlling the growth of carbon nanotubes is achieved by understanding and manipulating the delicate interplay between the catalyst, the carbon source, and the energy you provide.
Summary Table:
| Key Component | Role in CNT Growth |
|---|---|
| Carbon Source (e.g., Methane) | Provides raw carbon atoms for nanotube structure. |
| Catalyst (e.g., Fe, Co, Ni Nanoparticles) | Acts as a template; dictates nanotube diameter and initiates growth. |
| High Temperature (550–1050°C) | Decomposes carbon source and keeps catalyst active. |
| Growth Mechanism (Tip vs. Base) | Determines catalyst position (tip or base) based on substrate interaction. |
Ready to scale your carbon nanotube research or production? KINTEK specializes in precision lab equipment and consumables for advanced material synthesis, including Chemical Vapor Deposition (CVD) systems. Our expertise ensures you achieve optimal control over CNT growth parameters—from catalyst engineering to temperature management. Let us help you enhance yield, structure, and efficiency. Contact our experts today to discuss your laboratory needs!
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the process of MOCVD in nanotechnology? Precision Growth of Thin Films for Semiconductors
- What is the function of a high-temperature CVD tube furnace in 3D graphene foam prep? Master 3D Nanomaterial Growth
- How are CVD systems used for molecular sieve modification? Enhance Shape Selectivity & Para-Xylene Yield
- What are the methods of producing CNT? Scalable CVD vs. High-Purity Lab Techniques
- How do a precision thermostat and a platinum-rhodium thermocouple collaborate? Master AACVD Thermal Stability
- What is the sputtering process in physics? A Guide to Precision Thin-Film Deposition
- What is the principle of chemical vapor deposition? Unlock the Power of High-Purity Thin Film Deposition
- What is atomic layer deposition of a gas? Achieve Perfectly Uniform Thin Films with Atomic Precision



















