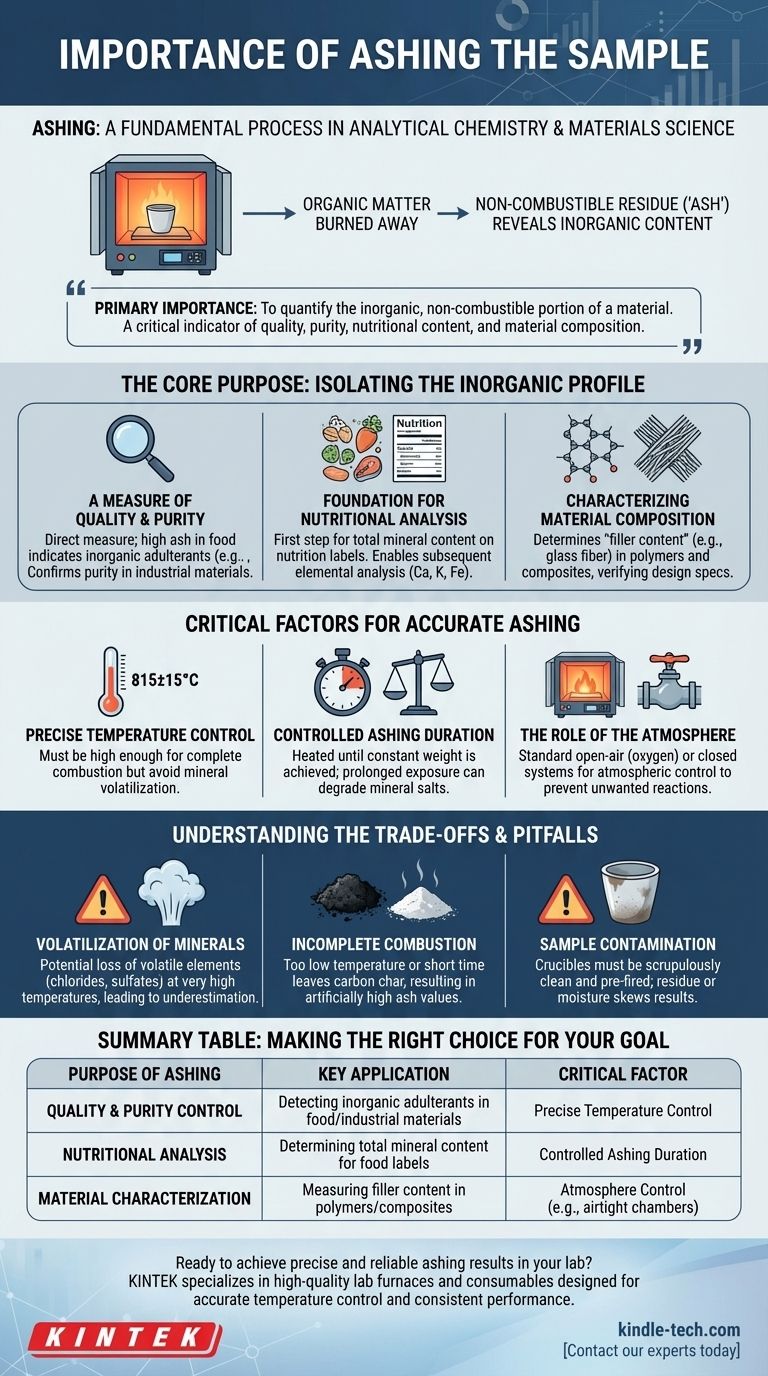
In analytical chemistry and materials science, ashing is a fundamental process used to determine the total mineral, or inorganic, content of a sample. By completely burning away all organic matter in a high-temperature furnace, the process leaves behind a non-combustible residue—the "ash." This resulting ash reveals a material's non-volatile composition, a critical data point for quality, characterization, and analysis.
The primary importance of ashing is to quantify the inorganic, non-combustible portion of a material. This single value serves as a critical indicator of quality, purity, nutritional content, and material composition across various industries.

The Core Purpose: Isolating the Inorganic Profile
Ashing is not simply about burning a sample; it is a carefully controlled method of decomposition. The data it provides serves several distinct purposes.
A Measure of Quality and Purity
For many products, the ash content is a direct measure of quality. A high ash value in a food product like flour or spices, for instance, can indicate the presence of inorganic adulterants such as sand or dirt.
In industrial materials, it confirms the purity of a substance by quantifying the non-essential inorganic matter.
Foundation for Nutritional Analysis
In food science, determining the total ash content is the first step in a complete nutritional profile. This total mineral content is a required value for many nutrition labels.
Furthermore, the resulting ash can be used for subsequent elemental analysis to determine the exact amounts of essential minerals like calcium, potassium, and iron.
Characterizing Material Composition
In fields like polymer science and composites manufacturing, ashing is used to determine the "filler content."
Fillers are inorganic materials (like glass fiber or calcium carbonate) added to a polymer to enhance its properties, such as strength or heat resistance. Ashing burns away the polymer matrix, leaving behind only the filler, allowing engineers to verify that the material meets its design specifications.
Critical Factors for Accurate Ashing
Achieving a meaningful result depends on precise control over the ashing process. The goal is complete combustion of organic material without altering the inorganic residue.
Precise Temperature Control
The chosen temperature is arguably the most critical variable. It must be high enough to ensure all organic matter burns away completely.
For example, a specific protocol might require a temperature of 815±15°C. Too low a temperature results in incomplete combustion, while too high a temperature can cause some minerals to volatilize and be lost, skewing the result.
Controlled Ashing Duration
The sample must be heated for a sufficient period to guarantee complete combustion. However, as noted in analytical standards, arbitrarily prolonging the ashing time is unfavorable.
Extended exposure to high temperatures can lead to the slow degradation or reaction of certain mineral salts, causing inaccurate measurements. The ideal duration achieves a constant weight, indicating the process is complete.
The Role of the Atmosphere
For most standard tests, ashing is done in a furnace open to the air (oxygen). However, some analyses require more control.
Using a closed system with an airtight chamber allows for atmospheric control. This is vital when the sample contains elements that might react with oxygen at high temperatures, which could alter their chemical form and mass.
Understanding the Trade-offs and Pitfalls
While the process is straightforward in principle, several factors can compromise the accuracy of the results. Awareness of these issues is essential for proper interpretation.
Volatilization of Minerals
One of the most significant challenges is the potential loss of volatile inorganic elements. Minerals like chlorides, sulfates, and some metallic oxides can be lost at very high temperatures, leading to an underestimation of the true mineral content.
This is why standardized methods specify precise temperatures—they represent a balance between complete combustion and minimal mineral loss.
Incomplete Combustion
The opposite problem is incomplete combustion, which occurs if the temperature is too low or the time is too short.
This leaves behind carbon char mixed with the ash, leading to an artificially high and incorrect ash value. The appearance of a pure, white or light gray ash is often a visual indicator of complete combustion.
Sample Contamination
The process is highly sensitive to external contamination. The crucibles used to hold the samples must be scrupulously clean and pre-fired to a constant weight. Any residue or moisture in the crucible will be incorrectly counted as part of the sample's ash content.
Making the Right Choice for Your Goal
To ensure your ashing procedure yields meaningful data, align your method with your analytical objective.
- If your primary focus is routine quality control: Standardize your temperature and time rigorously to ensure consistency and comparability between batches.
- If your primary focus is nutritional analysis: Follow established protocols (such as AOAC or ISO methods) precisely, as the goal is to obtain an accurate, legally defensible value.
- If your primary focus is materials characterization: Consider if a controlled atmosphere is necessary, as the interaction between the filler and the air at high temperatures can alter the results.
Ultimately, treating ashing not as a simple burn-off but as a controlled analytical procedure is the key to unlocking reliable insights into your material's core composition.
Summary Table:
| Purpose of Ashing | Key Application | Critical Factor |
|---|---|---|
| Quality & Purity Control | Detecting inorganic adulterants in food/industrial materials | Precise Temperature Control |
| Nutritional Analysis | Determining total mineral content for food labels | Controlled Ashing Duration |
| Material Characterization | Measuring filler content in polymers/composites | Atmosphere Control (e.g., airtight chambers) |
Ready to achieve precise and reliable ashing results in your lab?
KINTEK specializes in high-quality lab furnaces and consumables designed for accurate temperature control and consistent performance. Whether you're in food science, materials testing, or quality control, our equipment ensures your ashing procedures meet the highest standards.
Contact our experts today to find the perfect solution for your laboratory's needs!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How to cool a muffle furnace? Ensure Safety and Maximize Equipment Lifespan
- What is a muffle furnace used for in microbiology? Essential for Depyrogenation and Ashing
- What is the inside material of a muffle furnace? Choose the Right Lining for Your Application
- What 5 safety precautions should be taken when heating anything in the lab? Essential Rules for Lab Safety
- At what temperature does quartz soften? Understand the practical limits for lab equipment



















