Properly sealing an electrolytic cell is a non-negotiable requirement for three fundamental reasons: it ensures the accuracy of your results, protects the operator from chemical hazards, and prevents environmental contamination. A failure in the seal undermines the integrity of the entire experiment and introduces significant safety risks.
The seal of an electrolytic cell is not merely a lid; it is an active component that isolates the internal chemical environment. This control is essential for obtaining reliable data and ensuring the safety of both the operator and the surrounding workspace.

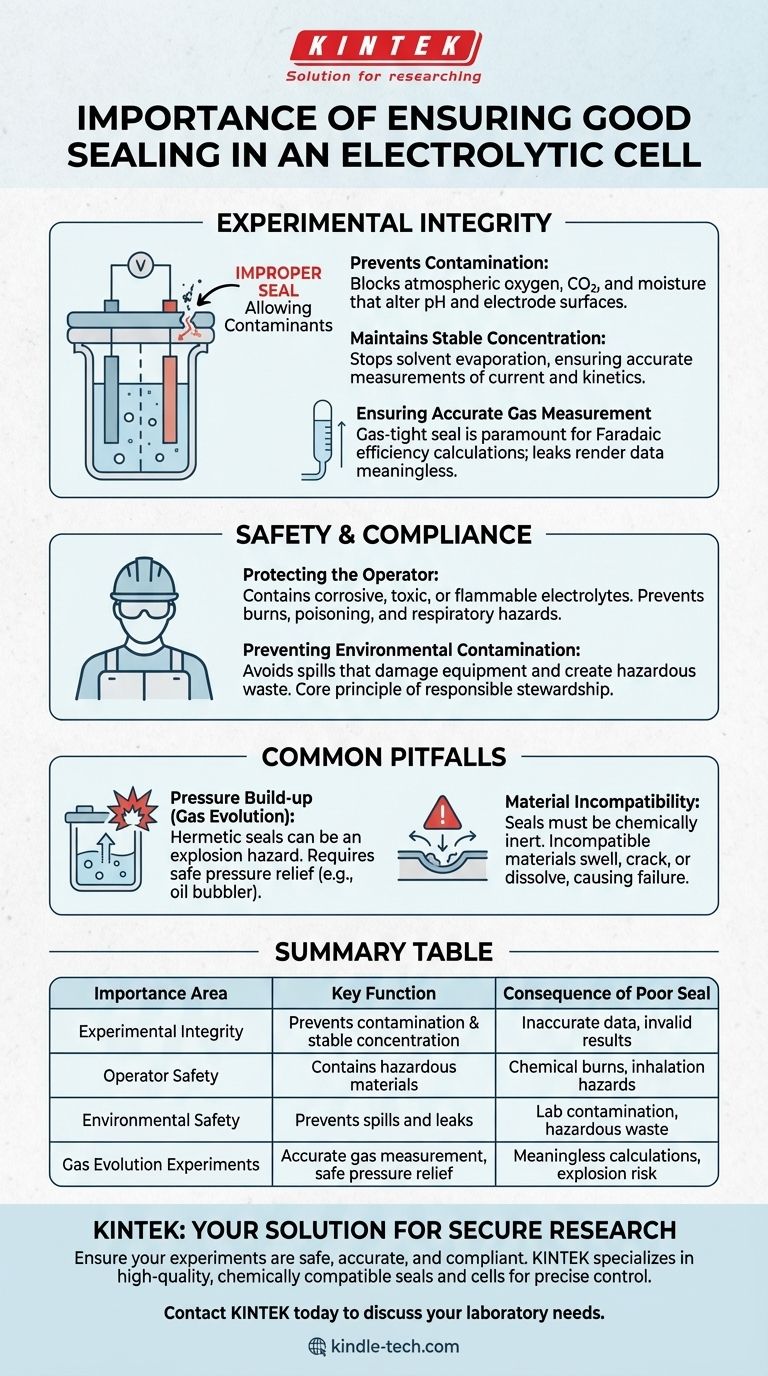
The Impact on Experimental Integrity
A compromised seal directly introduces uncontrolled variables into your experiment. This immediately invalidates any quantitative measurements and can lead to incorrect conclusions.
Preventing Contamination
Many electrochemical reactions are highly sensitive to the atmosphere. An improper seal allows oxygen, carbon dioxide, and moisture to enter the cell.
These contaminants can act as unwanted reactants, alter the pH of the electrolyte, or passivate electrode surfaces, fundamentally changing the reaction you intend to study.
Maintaining Stable Concentration
Electrolytes, especially those with volatile solvents, can evaporate over the course of an experiment. A poor seal accelerates this process.
This evaporation changes the concentration of your analyte and supporting electrolyte, which directly skews measurements of current, potential, and reaction kinetics.
Ensuring Accurate Gas Measurement
In experiments that produce gaseous products, such as water electrolysis, a gas-tight seal is paramount.
Any leak makes it impossible to accurately collect and measure the evolved gas, rendering calculations of Faradaic efficiency and production rates meaningless.
The Critical Role in Safety and Compliance
Beyond data accuracy, the primary function of a seal is containment. Failure to contain the cell's contents can have immediate and severe consequences.
Protecting the Operator
Electrolytes are often corrosive, toxic, or flammable. A leak can lead to chemical burns from acids or bases, poisoning from organic solvents, or other direct physical harm.
Vapors from a leaking cell can also be inhaled, posing a significant respiratory hazard.
Preventing Environmental Contamination
Even small leaks contribute to environmental pollution. Spilled electrolytes can damage lab equipment, enter drainage systems, and create hazardous waste.
Proper sealing is a core principle of responsible chemical handling and laboratory stewardship.
Common Pitfalls to Avoid
Achieving a "good" seal involves more than just preventing leaks; it requires understanding the specific demands of your electrochemical system.
Pressure Build-up
For experiments that evolve gas, a hermetically sealed cell is a significant explosion hazard. As gas is produced, pressure builds internally.
A proper setup must include a safe outlet for this pressure, such as an oil bubbler or a dedicated gas collection line, to prevent catastrophic failure of the cell.
Material Incompatibility
The material used for the seal (e.g., O-rings, stoppers, gaskets) must be chemically inert to the electrolyte and solvent.
Using an incompatible material will cause it to swell, crack, or dissolve, leading to both seal failure and contamination of your experiment.
Making the Right Choice for Your Goal
The required quality of your seal is dictated by the objective of your experiment.
- If your primary focus is quantitative analysis: Your seal must be hermetic to prevent any atmospheric contamination or change in electrolyte concentration.
- If your primary focus is gas evolution (e.g., electrolysis): Your seal must be gas-tight but incorporate a method for safe pressure relief and accurate gas collection.
- If your primary focus is general electrochemistry with non-sensitive materials: Your seal's main purpose is to prevent spills and minimize evaporation for basic safety and operational consistency.
Ultimately, a proper seal transforms an electrolytic cell from a simple container into a controlled and reliable scientific instrument.
Summary Table:
| Importance Area | Key Function | Consequence of Poor Seal |
|---|---|---|
| Experimental Integrity | Prevents contamination & maintains stable concentration | Inaccurate data, invalid results |
| Operator Safety | Contains corrosive, toxic, or flammable materials | Chemical burns, poisoning, inhalation hazards |
| Environmental Safety | Prevents spills and leaks | Lab contamination, hazardous waste |
| Gas Evolution Experiments | Enables accurate gas measurement with safe pressure relief | Meaningless efficiency calculations, explosion risk |
Ensure your electrochemical experiments are safe, accurate, and compliant. KINTEK specializes in high-quality lab equipment and consumables, including chemically compatible seals and cells designed for precise control and operator protection. Let our experts help you select the right equipment for your specific application. Contact KINTEK today to discuss your laboratory needs and enhance your research integrity.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H Type Electrolytic Cell Triple Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol



















