At its core, an induction furnace heats metal without fire. It uses a powerful, rapidly alternating magnetic field generated by a copper coil to induce strong electrical currents, called eddy currents, directly within the conductive material. The material's natural resistance to these currents generates intense heat through a process known as Joule heating, causing it to melt rapidly from the inside out.
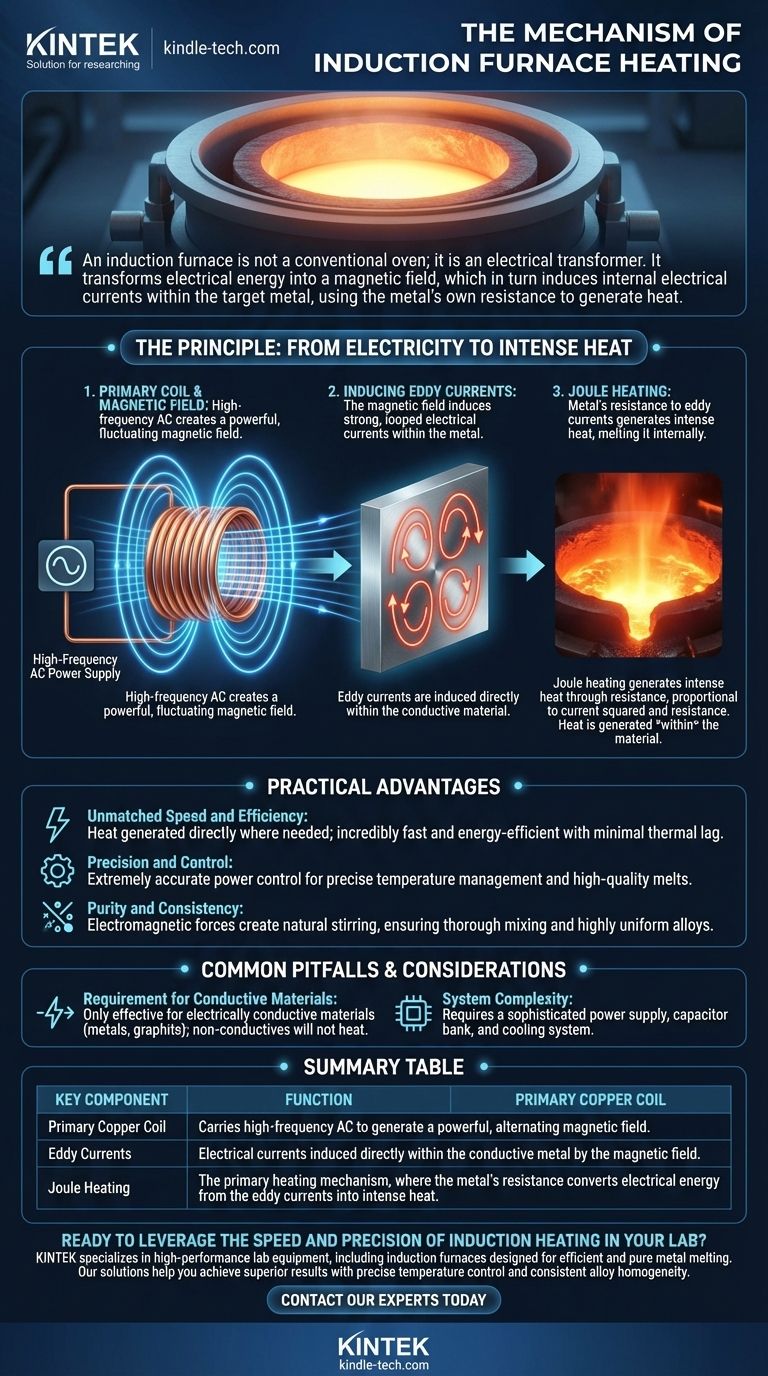
An induction furnace is not a conventional oven; it is an electrical transformer. It transforms electrical energy into a magnetic field, which in turn induces internal electrical currents within the target metal, using the metal's own resistance to generate heat.

The Principle: From Electricity to Intense Heat
The entire process is governed by the law of electromagnetic induction. Unlike a traditional furnace that applies external heat, an induction furnace makes the metal itself the source of the heat.
The Primary Coil and the Magnetic Field
An induction furnace begins with a coil, typically made of hollow copper tubing, that surrounds a crucible or the metal charge itself. A high-frequency alternating current (AC) from a specialized power supply is passed through this coil.
This flow of AC electricity generates a powerful and rapidly fluctuating magnetic field in the space within and around the coil.
Inducing Eddy Currents
When an electrically conductive material, like metal, is placed inside this alternating magnetic field, the field penetrates the material. This induces looped electrical currents within the metal.
These induced currents are known as eddy currents. The metal effectively becomes the secondary coil of a transformer, with the furnace's copper coil acting as the primary.
The Role of Joule Heating
Every metal has a degree of electrical resistance. As the strong eddy currents flow through the metal, they encounter this resistance, which converts the electrical energy directly into thermal energy, or heat.
This phenomenon is called Joule heating. The intensity of the heat is directly proportional to the square of the current and the resistance of the material, which is why the process can generate extremely high temperatures very quickly.
Why the Heat is 'Internal'
A critical distinction of this method is that heat is generated within the material. The furnace surfaces and crucible remain much cooler than the charge itself.
This internal generation leads to very rapid heating and melting, as energy is not wasted heating the air or walls of the furnace first.
Understanding the Practical Advantages
This unique heating mechanism provides several significant benefits that make it the preferred choice in many metallurgical applications.
Unmatched Speed and Efficiency
Because heat is generated directly where it is needed—inside the metal—the process is incredibly fast and energy-efficient. There is very little thermal lag or wasted energy compared to methods that rely on external combustion or heating elements.
Precision and Control
The power supplied to the coil can be controlled with extreme accuracy. This allows for precise temperature management, resulting in high-quality melts with minimal temperature differences between the core and the surface of the material.
Purity and Consistency
The electromagnetic forces generated by the eddy currents create a natural stirring action within the molten metal. This ensures that alloys are mixed thoroughly, leading to a highly uniform and homogenous final product without mechanical stirrers.
Common Pitfalls and Considerations
While powerful, the induction method is not a universal solution. Understanding its limitations is key to using it effectively.
Requirement for Conductive Materials
The entire principle is based on inducing electrical currents. Therefore, this method is only effective for heating materials that are electrically conductive, such as metals and graphite. Non-conductive materials like ceramics will not heat up directly.
System Complexity
An induction heating system consists of more than just the furnace. It requires a sophisticated power supply to generate the high-frequency current, a capacitor bank for power factor correction, and a cooling system for the copper coil, making it more complex than a simple fuel-fired furnace.
Making the Right Choice for Your Goal
Selecting a heating method depends entirely on the specific requirements of the material and the desired outcome.
- If your primary focus is high purity and precise alloy composition: The natural stirring effect and lack of combustion byproducts make induction the superior choice.
- If your primary focus is speed and energy efficiency: Direct internal heating is significantly faster and wastes less energy than heating an entire furnace chamber.
- If your primary focus is process control and repeatability: The high degree of temperature accuracy makes induction furnaces ideal for applications with tight metallurgical specifications.
Ultimately, understanding induction heating means recognizing that it treats the metal not as an object to be heated, but as an active component of the electrical circuit itself.
Summary Table:
| Key Component | Function |
|---|---|
| Primary Copper Coil | Carries high-frequency AC to generate a powerful, alternating magnetic field. |
| Eddy Currents | Electrical currents induced directly within the conductive metal by the magnetic field. |
| Joule Heating | The primary heating mechanism, where the metal's resistance converts electrical energy from the eddy currents into intense heat. |
Ready to leverage the speed and precision of induction heating in your lab?
KINTEK specializes in high-performance lab equipment, including induction furnaces designed for efficient and pure metal melting. Our solutions help you achieve superior results with precise temperature control and consistent alloy homogeneity.
Contact our experts today to discuss how an induction furnace can transform your metallurgical processes!
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Lab-Scale Vacuum Induction Melting Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- Vertical Laboratory Tube Furnace
People Also Ask
- How to clean a tube furnace? A Step-by-Step Guide for Safe and Effective Maintenance
- Why is a quartz tube furnace utilized in the thermal oxidation of MnCr2O4 coatings? Unlock Precise Selective Oxidation
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?
- What is the basic construction and temperature control mechanism of a laboratory tube furnace? Master Precision Heating for Your Lab
- What materials are used for the tubes in tube furnaces? A Guide to Selecting the Right Tube for Your Process



















