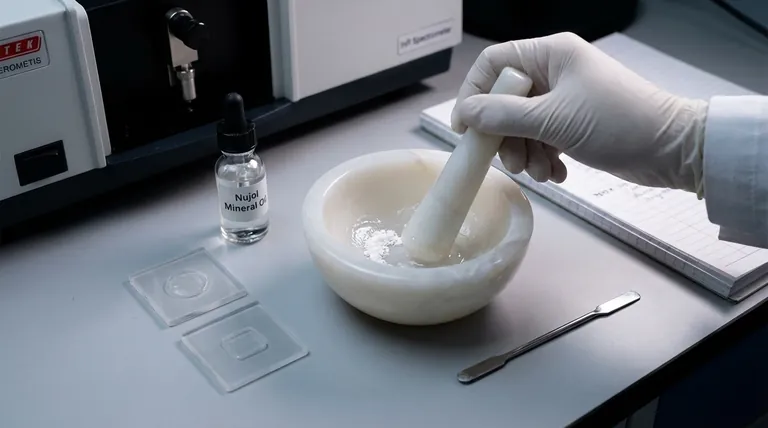
In analytical chemistry, the Nujol method is a common and rapid technique for preparing a solid sample for analysis by infrared (IR) spectroscopy. It involves finely grinding the solid material and mixing it with a few drops of Nujol, a high-purity mineral oil, to create a thick paste called a mull. This mull is then spread between two salt plates and placed in the spectrometer.
The core purpose of the Nujol method is to reduce light scattering from solid particles, producing a cleaner and more interpretable IR spectrum. Its primary trade-off is that the spectrum of Nujol itself—with its characteristic C-H absorption bands—will be superimposed onto your sample's spectrum.
The Core Problem: Why Solids Are Difficult for IR Spectroscopy
To understand why the Nujol method is necessary, we must first address the challenge of analyzing solid samples with infrared light.
The Challenge of Light Scattering
When an infrared beam hits a coarse, crystalline solid, the light doesn't just pass through it. Instead, the particles scatter the light in multiple directions.
This scattering effect is detrimental to the quality of the spectrum. It causes a sloping baseline and distorted, poorly defined absorption peaks, making the resulting data difficult or impossible to interpret accurately.
The Solution: Index Matching
The Nujol method solves this problem by a principle similar to refractive index matching. By grinding the solid into very fine particles (ideally smaller than the wavelength of the IR light) and suspending them in mineral oil, the amount of light scattering is dramatically reduced.
The oil coats the particles and fills the air gaps, creating a more uniform medium for the light to pass through. This allows the spectrometer's detector to measure the light that was absorbed by the sample's chemical bonds, not the light that was scattered away by its physical form.
How the Nujol Mull Method Works
The procedure is valued for its simplicity and speed, typically taking only a few minutes.
Step 1: Grinding the Sample
A small amount of the solid sample (typically 2-5 mg) is placed in a mortar, often made of agate, and ground thoroughly with a pestle. The goal is to produce a fine, flour-like powder.
Step 2: Creating the Mull
One or two drops of Nujol are added to the powder. The mixture is then ground further until it forms a uniform, translucent, and viscous paste with no visible solid particles. The consistency should be similar to that of a thick ointment.
Step 3: Mounting the Sample
A small dab of the mull is smeared onto the surface of a polished salt plate (commonly made of NaCl or KBr). A second salt plate is placed on top and gently rotated to spread the mull into a thin, even film.
This "sandwich" of salt plates containing the sample is then placed into a holder and inserted into the IR spectrometer for analysis.
Understanding the Trade-offs of Using Nujol
Like any analytical technique, the Nujol method has clear advantages and disadvantages that make it suitable for some applications but not others.
Advantage: Simplicity and Speed
The Nujol method is exceptionally fast and requires minimal equipment beyond a mortar, pestle, and salt plates. It is often the quickest way to get a qualitative survey spectrum of an unknown solid.
Advantage: Sample Integrity
The process is non-destructive and gentle. Unlike the KBr pellet method, it does not involve high pressures that could potentially alter the crystalline structure of the sample.
Disadvantage: Inherent Spectral Interference
This is the most significant drawback. Nujol is a mixture of long-chain alkanes (hydrocarbons), and it has its own IR absorption bands. Strong peaks from its C-H bonds will always appear in your spectrum around:
- 2924 cm⁻¹ (C-H stretch)
- 1462 cm⁻¹ (C-H bend)
- 1377 cm⁻¹ (C-H bend)
If your sample has important functional groups that absorb in these regions, Nujol will obscure them.
Disadvantage: Potential for Incomplete Data
Because of the interference, a Nujol mull cannot provide a complete picture of the molecule. To see the C-H regions clearly, chemists often prepare a second mull using a complementary agent like Fluorolube—a fluorinated polymer that absorbs where Nujol is transparent, and vice versa.
How to Apply This to Your Project
Your choice of sample preparation method depends entirely on the information you need from your analysis.
- If your primary focus is a fast, qualitative survey: The Nujol method is an excellent first choice to quickly identify major functional groups outside of the C-H regions.
- If your primary focus is a complete, interference-free spectrum: Preparing a KBr pellet is the gold standard, as KBr is transparent through the entire mid-IR range. This method is more time-consuming and sensitive to moisture.
- If your primary focus is the C-H stretching or bending regions: You must use an alternative like a KBr pellet or a Fluorolube mull, as Nujol will completely obscure this data.
Ultimately, mastering the Nujol method is about understanding and accepting the fundamental trade-off between analytical speed and absolute spectral purity.
Summary Table:
| Aspect | Details |
|---|---|
| Purpose | Prepare solid samples for IR spectroscopy by reducing light scattering. |
| Key Advantage | Fast, simple, and non-destructive preparation. |
| Main Limitation | Nujol's C-H absorption bands (2924, 1462, 1377 cm⁻¹) obscure sample peaks. |
| Ideal For | Quick qualitative survey of functional groups outside the C-H region. |
| Alternative Method | KBr pellet for a complete, interference-free spectrum. |
Need to optimize your lab's IR spectroscopy workflow?
The Nujol method is just one technique for solid sample analysis. At KINTEK, we specialize in providing the right lab equipment and consumables—from high-quality salt plates (NaCl, KBr) and mulling agents to robust IR spectrometers—to ensure your analytical results are accurate and reliable.
Let our experts help you select the perfect tools for your specific application. Contact us today to discuss your laboratory needs and discover how KINTEK can enhance your research capabilities.
Visual Guide

Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Three-dimensional electromagnetic sieving instrument
- Infrared Heating Quantitative Flat Plate Press Mold
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Shaking Incubators for Diverse Laboratory Applications
People Also Ask
- How does a high-precision oven contribute to the post-processing of hydrothermal oxidation products? Ensure Data Purity
- What are the advantages of belt filter press? Achieve High-Volume Dewatering with Low Operational Cost
- What are the precautions to be taken for heating of a substance in the laboratory? Ensure Safety and Prevent Accidents
- How does temperature affect the mechanical properties of materials? Avoid Brittle Fracture & Creep Failure
- Can you harden non-ferrous metals? Yes, with the right methods for aluminum, copper, and titanium
- What is magnetron plasma? A Guide to High-Efficiency Thin-Film Deposition
- Why KBr is used to prepare samples for FTIR analysis? Unlock Clear, High-Quality Spectra
- What are laser sintering methods? Unlock Complex 3D Printing with Powder Bed Fusion










