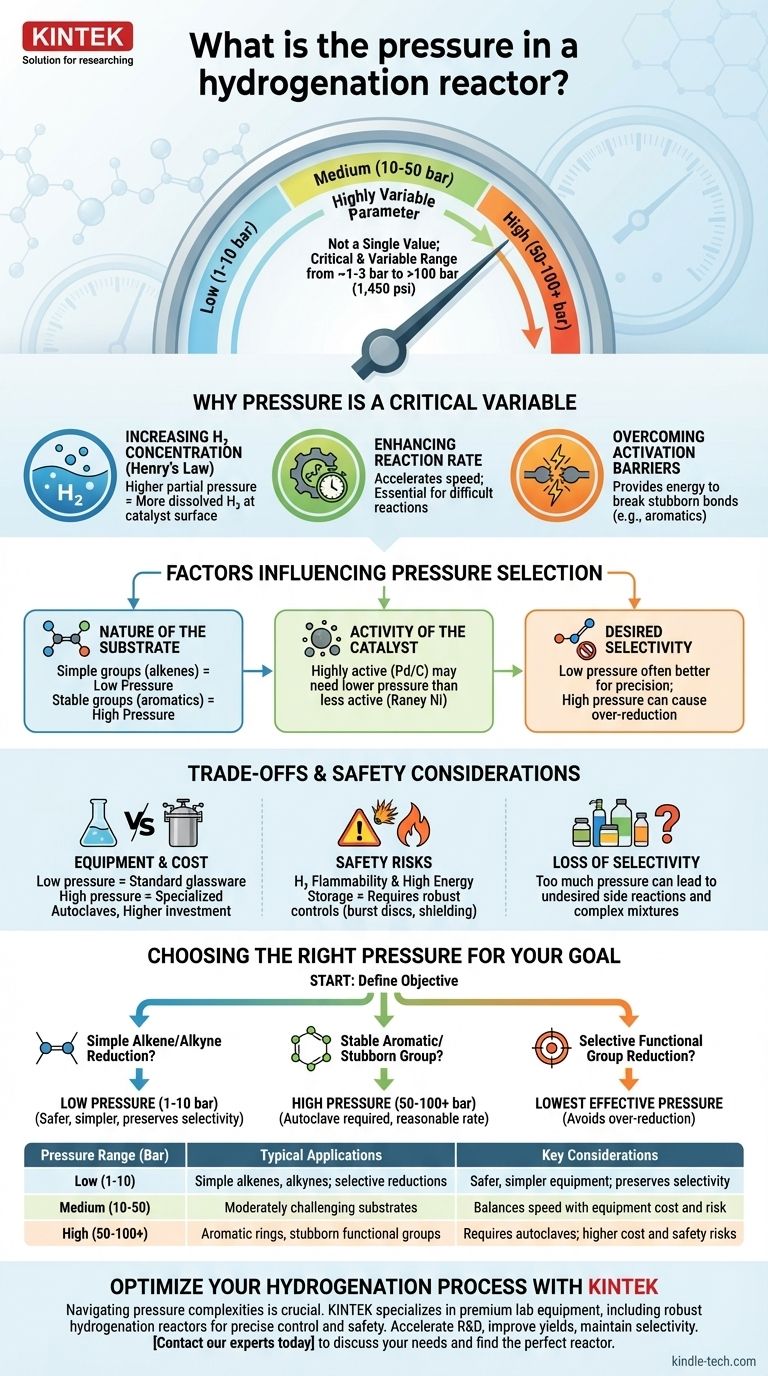
The pressure in a hydrogenation reactor is not a single value but a critical, highly variable parameter. It can range from just above atmospheric pressure (~1-3 bar) for simple reactions to well over 100 bar (1,450 psi) for more challenging transformations. The specific pressure is deliberately chosen to control the reaction's speed, efficiency, and outcome based on the molecule being hydrogenated and the catalyst being used.
The choice of pressure for hydrogenation is a fundamental trade-off. Higher pressures increase reaction rates by forcing more hydrogen gas into the solution, but this comes at the cost of more expensive equipment, increased safety risks, and a potential loss of chemical selectivity.
Why Pressure Is a Critical Variable
Pressure is not just a setting; it is a primary lever for controlling the chemical environment inside the reactor. Its influence stems from fundamental principles of physical chemistry.
Increasing Hydrogen Concentration
At its core, hydrogenation is a reaction between a substrate dissolved in a liquid and hydrogen, which is a gas. According to Henry's Law, increasing the partial pressure of hydrogen gas above the liquid directly increases the concentration of hydrogen molecules dissolved in the solvent.
This is the most crucial effect of pressure. More dissolved hydrogen means more reactant is available at the surface of the catalyst where the reaction actually happens.
Enhancing Reaction Rate
Chemical reaction rates are dependent on the concentration of the reactants. By increasing the amount of dissolved hydrogen, you are directly accelerating the rate of the hydrogenation.
For difficult or slow reactions, applying high pressure is often the only practical way to make the process complete in a reasonable amount of time.
Overcoming Activation Barriers
Some chemical bonds, like those in aromatic rings (e.g., benzene), are exceptionally stable and resistant to being broken. Hydrogenating these "stubborn" substrates requires significant energy.
High hydrogen pressure helps overcome this energy barrier, enabling the catalyst to perform the reduction efficiently. For these molecules, low-pressure conditions would result in little to no reaction.
Factors Influencing Pressure Selection
The ideal pressure is not universal. It is determined by a careful analysis of the specific chemical system.
The Nature of the Substrate
This is the most important factor. Simple, reactive functional groups like alkenes (C=C) or alkynes (C≡C) are easily reduced and often only require low pressure (1-10 bar).
In contrast, stable groups like aromatic rings, amides, or carboxylic acids are much harder to reduce and frequently demand high pressures (50-100+ bar) to proceed.
The Activity of the Catalyst
Catalysts have varying levels of intrinsic activity. For a given reaction, a highly active catalyst like Palladium on Carbon (Pd/C) might achieve a full conversion at a lower pressure than a less active one like Raney Nickel.
The choice of catalyst and pressure are therefore deeply intertwined.
Desired Selectivity
Sometimes, the goal is not to reduce everything, but to selectively reduce one functional group while leaving another untouched.
In these cases, lower pressure is often advantageous. High pressure can act like a sledgehammer, causing "over-reduction" and destroying the desired selectivity. A gentler, low-pressure approach allows for more fine-tuned chemical transformations.
Understanding the Trade-offs and Safety Considerations
Choosing to use high pressure is a significant decision with major practical implications. It introduces complexity, cost, and risk that must be carefully managed.
Equipment and Cost
Low-pressure hydrogenations can often be done in standard laboratory glassware. High-pressure reactions, however, require a specialized, thick-walled steel vessel known as an autoclave or pressure reactor.
These reactors, along with the associated high-pressure gas lines, regulators, and safety monitoring systems, represent a significant financial and infrastructure investment.
Safety Risks
Hydrogen gas is extremely flammable and can form explosive mixtures with air. At high pressure, the amount of stored energy is immense, amplifying the risk of a leak or vessel failure.
Proper engineering controls, such as burst discs, remote operation, blast shields, and robust ventilation, are not optional—they are essential for safe operation. This is why a dedicated, well-designed reactor and gas-dosing system is critical for reproducibility and safety.
Loss of Selectivity
As mentioned, while high pressure speeds up a desired reaction, it can also accelerate undesired side reactions. If your molecule has multiple reducible groups, applying too much pressure may cause you to lose your intended product, resulting in a complex mixture that is difficult to purify.
Choosing the Right Pressure for Your Goal
Your choice of pressure should be dictated by your specific objective. A methodical approach, starting with literature precedents for similar molecules, is always the best practice.
- If your primary focus is reducing a simple, unhindered alkene or alkyne: Start with low pressure (1-10 bar), as it is often sufficient, safer, and requires less specialized equipment.
- If your primary focus is reducing a stable aromatic ring or a stubborn functional group: You will likely need high pressure (50-100+ bar) and a more robust reactor to achieve a reasonable reaction rate.
- If your primary focus is selectively reducing one functional group over another: Use the lowest effective pressure possible, as higher pressures can lead to over-reduction and a loss of selectivity.
Ultimately, pressure is a powerful tool that, when understood and controlled, allows a chemist to precisely tune the outcome of a hydrogenation reaction.
Summary Table:
| Pressure Range (Bar) | Typical Applications | Key Considerations |
|---|---|---|
| Low (1-10 bar) | Simple alkenes, alkynes; selective reductions | Safer, simpler equipment; preserves selectivity |
| Medium (10-50 bar) | Moderately challenging substrates | Balances speed with equipment cost and risk |
| High (50-100+ bar) | Aromatic rings, stubborn functional groups | Requires autoclaves; higher cost and safety risks |
Optimize Your Hydrogenation Process with KINTEK
Navigating the complexities of hydrogenation pressure is crucial for achieving your desired reaction outcomes safely and efficiently. Whether you are working on selective reductions at low pressure or challenging transformations requiring a high-pressure autoclave, having the right equipment is fundamental.
KINTEK specializes in premium lab equipment and consumables, including robust hydrogenation reactors designed for precise pressure control and maximum safety. Our solutions help chemists like you accelerate R&D, improve yields, and maintain critical selectivity.
Let us help you enhance your laboratory's capabilities. Contact our experts today to discuss your specific hydrogenation needs and find the perfect reactor for your research.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- Why are high-pressure autoclaves essential for preparing bio-based polyamide curing agents from dimeric acid?
- How does a high-pressure reactor demonstrate its value in accelerated aging? Predict Catalyst Durability Fast
- What is the purpose of using a high-temperature hydrothermal reactor? Enhance Iodine@Activated Carbon Cathode Synthesis
- What roles do autoclaves play in MFI zeolite synthesis? Master Hydrothermal Crystalline Growth
- What is the purpose of using high-purity argon gas in a high-pressure reactor? Ensure Precise Corrosion Test Data



















