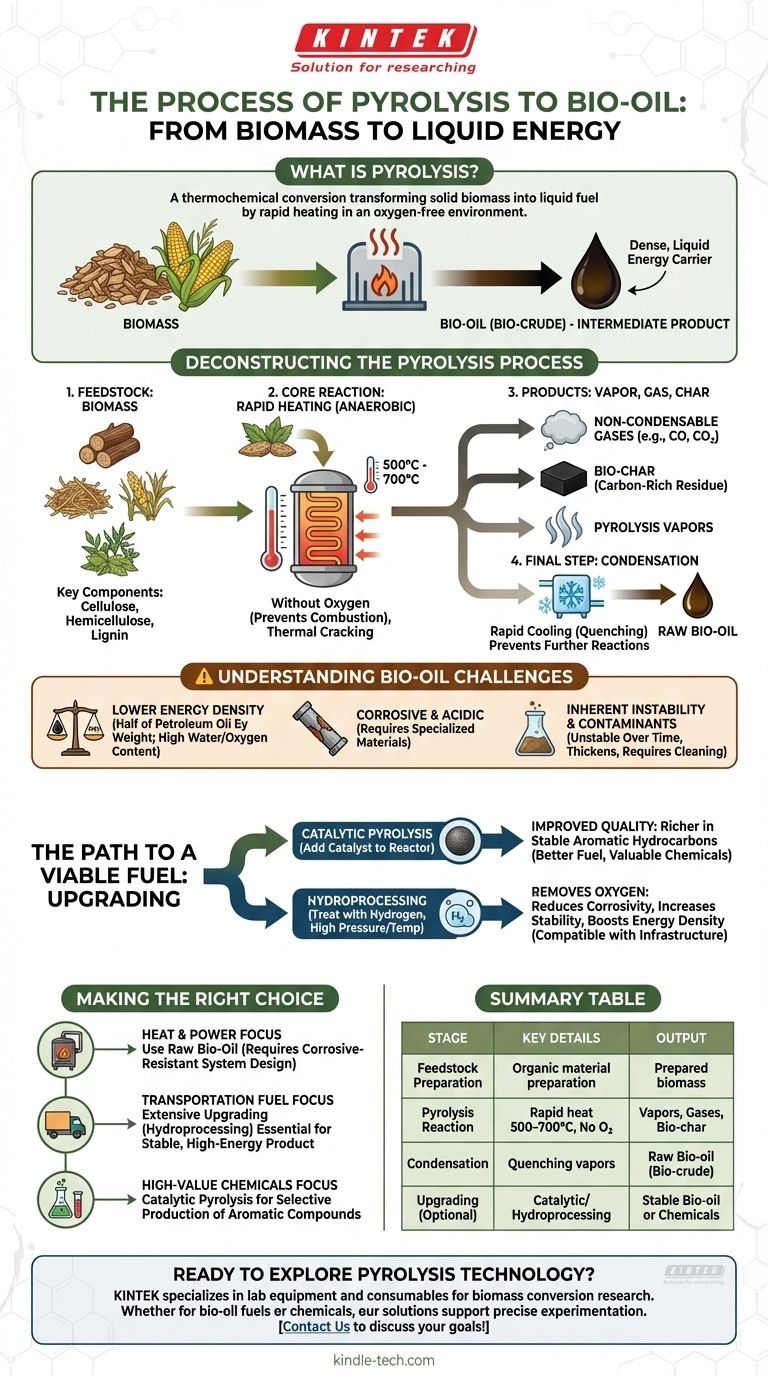
In essence, pyrolysis is a thermochemical conversion process that transforms solid biomass into a liquid fuel. It involves rapidly heating organic material, such as wood or agricultural waste, to high temperatures in an environment with little to no oxygen. This process breaks down the complex polymers in the biomass into vapors, which are then quickly cooled and condensed to form a dark, viscous liquid known as bio-oil.
The core purpose of pyrolysis is to convert bulky, solid biomass into a dense, liquid energy carrier. However, this resulting "bio-crude" is not a direct replacement for petroleum; it is an intermediate product that requires significant processing to become a stable, compatible fuel.

Deconstructing the Pyrolysis Process
To understand bio-oil, we must first understand the precise, multi-stage process that creates it. It is a carefully controlled thermal decomposition, not simple burning.
The Feedstock: Starting with Biomass
The process begins with organic material, or biomass. This can include wood chips, crop residues, or other plant-based matter.
The key components within the biomass that are targeted for conversion are cellulose, hemicellulose, and lignin.
The Core Reaction: Rapid Heating Without Oxygen
The biomass is fed into a reactor and heated very quickly to temperatures between 500°C and 700°C.
Crucially, this occurs in an oxygen-free (anaerobic) atmosphere. The absence of oxygen prevents the biomass from combusting and instead forces it to thermally break down, or "crack."
The Products: Vapor, Gas, and Char
This rapid thermal cracking decomposes the biomass into three primary outputs.
First are the pyrolysis vapors, which contain the condensable compounds that will form the bio-oil. Second is a stream of non-condensable gases (like CO and CO2), and third is a solid, carbon-rich residue called bio-char.
The Final Step: Condensation to Bio-Oil
After the solid bio-char is separated, the hot pyrolysis vapors are passed through a condenser.
Here, they are rapidly cooled, or quenched. This rapid condensation prevents further chemical reactions and transforms the vapors into the liquid bio-oil.
Understanding the Trade-offs and Challenges
While pyrolysis is a powerful conversion technology, the resulting bio-oil presents several significant challenges that prevent its widespread, direct use. Acknowledging these limitations is critical for any practical application.
Lower Energy Density
The heating value of raw bio-oil is only about half that of conventional petroleum-based heating oil by weight. This is primarily due to its high oxygen and water content.
Corrosive and Acidic Nature
Bio-oil is highly acidic and corrosive to common construction metals like steel. This requires specialized storage tanks, pumps, and engine components, adding significant cost and complexity.
Inherent Instability and Contaminants
Raw bio-oil is chemically unstable and can thicken or even solidify over time, especially when heated. It also contains contaminants that must be removed before it can be used in most engines or refineries.
The Path to a Viable Fuel: Upgrading Bio-Oil
Because of its challenging properties, raw bio-oil is best viewed as a "bio-crude" that must be upgraded. Several methods exist to stabilize it and improve its quality.
Catalytic Pyrolysis
By introducing a catalyst, such as HZSM-5, directly into the pyrolysis reactor, the quality of the initial vapors can be dramatically improved. This process can produce a bio-oil richer in stable aromatic hydrocarbons, making it a better fuel or a source for valuable chemicals.
Hydroprocessing
This is a critical upgrading technique where bio-oil is treated with hydrogen under pressure and high temperature. Hydroprocessing removes oxygen, which reduces the oil's corrosivity, increases its stability, and significantly boosts its energy density, making it more compatible with existing fuel infrastructure.
Making the Right Choice for Your Goal
The viability of bio-oil depends entirely on the intended application and a clear understanding of the necessary post-processing.
- If your primary focus is creating a direct source for heat and power: Raw bio-oil can be used in specially designed industrial boilers or furnaces, but you must account for its corrosive properties and lower energy content in your system design.
- If your primary focus is producing a transportation-grade fuel: Extensive upgrading through processes like hydroprocessing is non-negotiable to create a stable, high-energy-density product that can be blended with conventional fuels.
- If your primary focus is developing high-value chemicals: Catalytic pyrolysis offers a pathway to selectively produce valuable aromatic compounds, shifting the economic model away from bulk fuel and toward specialty chemical production.
Ultimately, pyrolysis is a highly effective technology for liquefying biomass, but the journey from raw bio-crude to a finished, marketable product requires a clear-eyed assessment of its inherent challenges and the upgrading pathways needed to overcome them.
Summary Table:
| Process Stage | Key Details | Output |
|---|---|---|
| Feedstock Preparation | Organic material like wood chips or crop residues. | Prepared biomass |
| Pyrolysis Reaction | Rapid heating to 500–700°C in an oxygen-free environment. | Vapors, gases, bio-char |
| Condensation | Quenching vapors to form liquid bio-oil. | Raw bio-oil (bio-crude) |
| Upgrading (Optional) | Catalytic pyrolysis or hydroprocessing for stabilization. | Stable bio-oil or chemicals |
Ready to explore pyrolysis technology for your lab or pilot project? KINTEK specializes in lab equipment and consumables for biomass conversion research, including reactors and analysis tools. Whether you're developing bio-oil fuels or high-value chemicals, our solutions support precise, efficient experimentation. Contact us today to discuss how we can help you achieve your biomass conversion goals!
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- Why does heating increase temperature? Understanding the Molecular Dance of Energy Transfer
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- How do you clean a tubular furnace tube? A Step-by-Step Guide to Safe and Effective Maintenance
- What is quartz tube heating? Achieve Instant, Targeted Heat with Infrared Radiation
- What is the difference between upflow and horizontal furnace? Find the Perfect Fit for Your Home's Layout



















