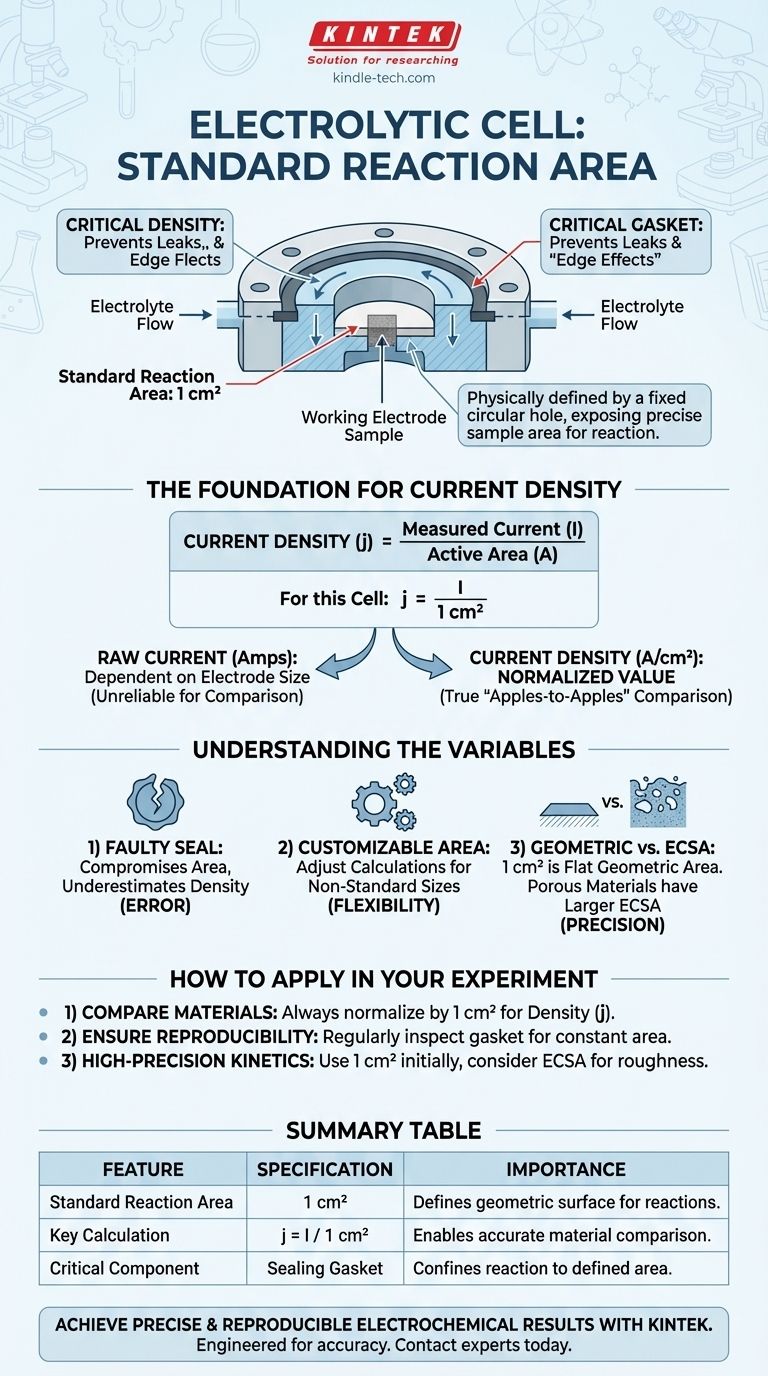
Based on the provided specifications, the standard reaction area of the working electrode in this electrolytic cell is 1 square centimeter (1 cm²). This area is physically defined by a fixed circular hole at the bottom of the cell, through which the working electrode sample is exposed to the electrolyte.
The question about the electrode's area points to a more fundamental need: ensuring experimental results are accurate and comparable. The fixed 1 cm² area is not just a dimension; it is the foundation for calculating current density, the universal metric for evaluating electrochemical performance.

The Role of the Fixed Reaction Area
The physical design of the cell is intentionally engineered to control the variables of your experiment. The defined opening for the working electrode is arguably the most critical feature.
Defining the Active Surface
The 1 cm² hole acts as a mask, exposing only a precise and known area of your sample material (the working electrode) to the electrolyte. All electrochemical reactions are confined to this specific surface.
This design ensures that the geometric surface area involved in the reaction is constant from one experiment to the next, which is essential for reproducibility.
The Critical Function of the Gasket
A gasket is used to create a tight seal around the perimeter of this 1 cm² hole. Its purpose is twofold: to prevent the electrolyte from leaking and to stop the reaction from spreading beyond the defined area.
Without a proper seal, "crevice corrosion" or "edge effects" can occur, leading to an ill-defined reaction area and rendering your measurements inaccurate.
From Area to Current Density: The True Metric
Knowing the reaction area is the first step. The ultimate goal is to use it to calculate a far more insightful value: current density.
What is Current Density?
Current density is the total electrical current measured in an experiment divided by the active area of the electrode. It is typically expressed in amperes per square centimeter (A/cm²) or milliamperes per square centimeter (mA/cm²).
For this cell, the calculation is straightforward: Current Density (j) = Measured Current (I) / 1 cm².
Why It Matters More Than Raw Current
Simply measuring the total current (in Amps) is insufficient for comparison, as this value will naturally be larger for a larger electrode. It doesn't reflect the intrinsic efficiency or activity of the electrode material itself.
By normalizing the current to the area, you create a value—current density—that allows for a true apples-to-apples comparison between different materials, catalysts, or experimental conditions, regardless of minor variations in sample size.
Understanding the Trade-offs and Considerations
While the design provides a standard, several factors must be managed to ensure the integrity of your results.
The Impact of a Faulty Seal
A worn-out, improperly seated, or chemically degraded gasket is a primary source of experimental error. If the seal is compromised, the actual reaction area may be larger than the assumed 1 cm², causing you to underestimate the true current density.
The "Customizable" Area Option
The reference notes that the hole size can be customized. While 1 cm² is a convenient standard, a different area might be necessary for certain applications.
For instance, a smaller area might be used for highly expensive or scarce materials, while a larger area might be required for materials with very low conductivity. If you use a custom area, all current density calculations must be adjusted accordingly.
Geometric vs. Electrochemical Surface Area (ECSA)
It is critical to distinguish between the geometric area (the 1 cm² flat circle) and the electrochemical surface area (ECSA). If your electrode material is porous, rough, or nanostructured, its true surface area at the microscopic level will be much larger than 1 cm².
For most routine comparisons, normalizing by the geometric area is sufficient. For advanced kinetic studies, however, researchers often use techniques like capacitance measurements to estimate the ECSA for a more precise understanding of catalytic activity.
How to Apply This to Your Experiment
Use the standard area as your baseline for generating reliable and meaningful data.
- If your primary focus is comparing different materials: Always normalize your measured current by the 1 cm² area to calculate and report current density (j).
- If your primary focus is ensuring reproducibility: Regularly inspect the gasket for wear and ensure it is seated correctly before every experiment to maintain a constant 1 cm² reaction area.
- If your primary focus is high-precision kinetics: Use the 1 cm² geometric area for initial calculations, but consider measuring the true Electrochemical Surface Area (ECSA) if your material has a high degree of surface roughness.
Ultimately, using the defined reaction area to calculate current density is the key to producing repeatable and comparable electrochemical data.
Summary Table:
| Feature | Specification | Importance |
|---|---|---|
| Standard Reaction Area | 1 cm² | Defines the geometric surface area for all reactions. |
| Key Calculation | Current Density (j) = Measured Current (I) / 1 cm² | Enables accurate comparison of different materials. |
| Critical Component | Sealing Gasket | Prevents leaks and ensures the reaction is confined to the 1 cm² area. |
Achieve precise and reproducible electrochemical results with KINTEK.
Our electrolytic cells are engineered with a precisely defined 1 cm² reaction area to eliminate variables and provide the foundation for accurate current density calculations. This ensures your data on catalyst performance, material efficiency, and reaction kinetics is reliable and comparable.
KINTEK specializes in high-quality lab equipment and consumables for all your laboratory needs. Let our expertise support your research.
Contact our experts today to discuss how our equipment can enhance your electrochemical workflow.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- How should the five-port water bath electrolytic cell be operated during an experiment? Master Precise Control for Reliable Results



















