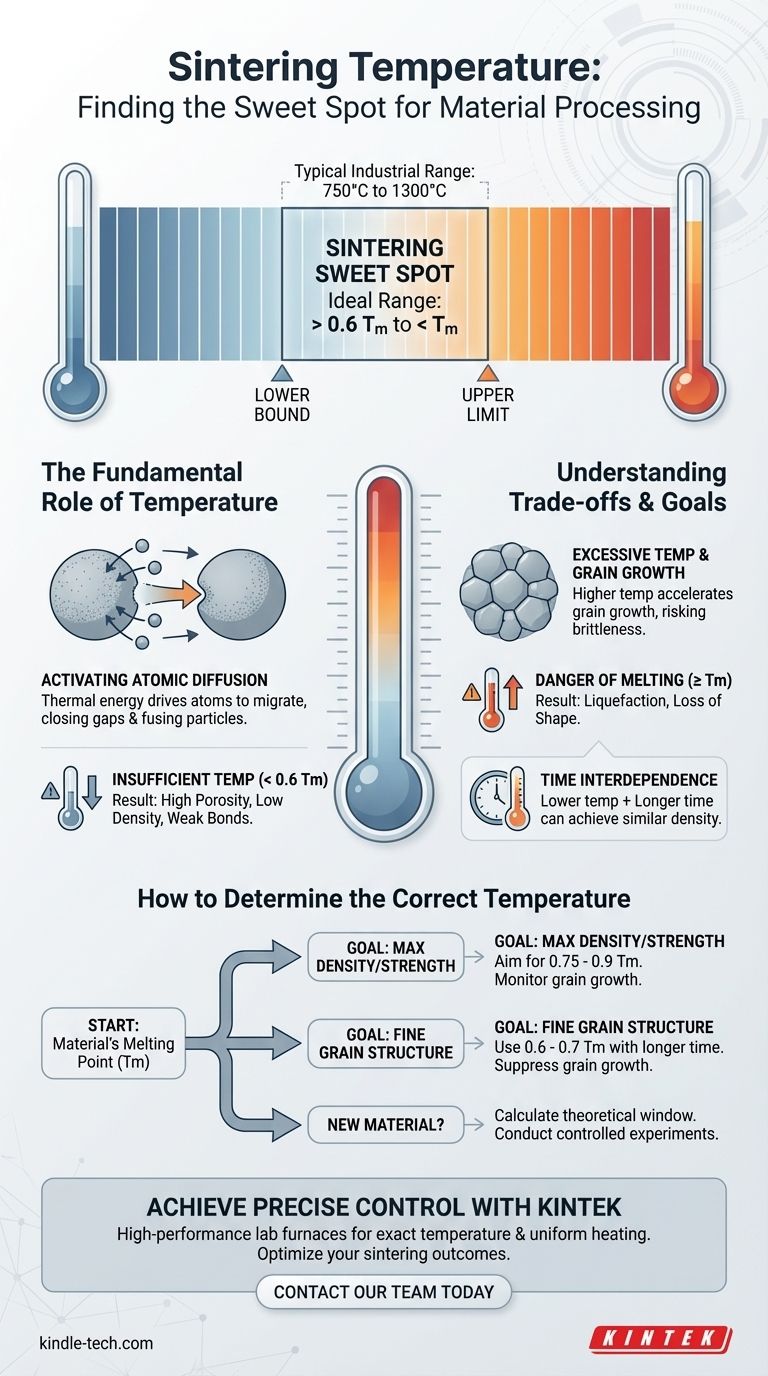
In practice, the ideal sintering temperature is not a single value but a carefully selected point within a specific range, governed by the material's fundamental properties. For most materials, the process is conducted at a temperature greater than 60% of the absolute melting temperature (Tm), which often falls within a general industrial range of 750°C to 1300°C.
The central challenge of sintering is finding the "sweet spot": a temperature high enough to activate atomic diffusion and fuse particles together, but low enough to prevent melting and undesirable microstructural changes like excessive grain growth.

The Fundamental Role of Temperature
Sintering is a thermally activated process. Understanding how temperature drives the underlying mechanisms is key to controlling the outcome.
Activating Atomic Diffusion
Sintering works by motivating atoms to move, closing the gaps between powder particles and increasing the material's density.
Temperature provides the necessary thermal energy for this atomic diffusion to occur at a practical rate. As particles heat up, atoms migrate across their boundaries, forming "necks" that grow and eventually consolidate the loose powder into a solid mass.
Establishing the Lower Temperature Bound
There is a minimum thermal energy required for effective diffusion. A widely accepted rule of thumb is that the sintering temperature should be at least 0.6 times the material's melting temperature (Tm).
Below this threshold, atomic movement is too slow, and the consolidation process becomes impractically long or fails to achieve the desired density.
Defining the Upper Temperature Limit
The absolute upper limit for any sintering process is the material's melting point.
If the temperature reaches or exceeds this point, the material will begin to liquefy, losing its shape and structural integrity. Sintering is, by definition, a solid-state process that must occur below the melting point.
Understanding the Trade-offs
Choosing a temperature is an exercise in balancing competing factors. The temperature you select directly influences the final properties of the component.
The Risk of Insufficient Temperature
Operating at too low a temperature results in incomplete sintering.
This leads to a final part with high porosity, low density, and poor mechanical properties, such as low strength and fracture toughness. The bonds between the original particles will be weak.
The Danger of Excessive Temperature
While higher temperatures increase the rate of densification, they also accelerate another phenomenon: grain growth.
Excessively large grains can make a material brittle, reducing its strength and toughness. Pushing the temperature too high, even if below the melting point, can therefore be counterproductive to achieving optimal mechanical performance.
The Influence of Time
Temperature and time are interdependent variables in sintering.
A lower temperature can sometimes achieve the same densification as a higher temperature if the processing time is significantly extended. This is a common strategy used to refine grain structure and control final properties with high precision.
How to Determine the Correct Temperature
Your specific goal will determine the ideal point within the viable temperature window. Use the material's melting point as your starting reference and adjust based on your primary objective.
- If your primary focus is achieving maximum density and strength: Aim for a higher temperature within the sintering window (e.g., 0.75 - 0.9 Tm) to maximize the rate of diffusion, but carefully monitor for the onset of rapid grain growth.
- If your primary focus is controlling fine grain structure for toughness: Use a lower temperature (e.g., 0.6 - 0.7 Tm) and compensate with a longer hold time to achieve density while suppressing excessive grain growth.
- If you are working with a new or uncharacterized material: Start by calculating the theoretical window based on its melting point (Tm), then conduct a series of controlled experiments to observe densification and microstructural evolution at different temperatures.
Ultimately, temperature is the primary lever you use to steer the sintering process toward the desired final material properties.
Summary Table:
| Factor | Role in Sintering | Temperature Guideline |
|---|---|---|
| Lower Bound | Activates atomic diffusion | ≥ 0.6 x Melting Temp (Tm) |
| Upper Bound | Prevents melting & excessive grain growth | < Melting Temp (Tm) |
| Goal: Max Density/Strength | Maximizes diffusion rate | 0.75 - 0.9 Tm |
| Goal: Fine Grain Structure | Suppresses grain growth | 0.6 - 0.7 Tm (with longer time) |
Achieve precise control over your sintering process with KINTEK.
Choosing the right temperature is critical for developing materials with the exact density, strength, and microstructure you need. KINTEK specializes in high-performance lab furnaces that deliver the precise temperature control and uniform heating required for reliable sintering results.
Our experts can help you select the ideal equipment for your specific materials and research goals. Let's optimize your sintering outcomes together—contact our team today for a personalized consultation.
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Mesh belt controlled atmosphere furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the difference between calcining and roasting? A Guide to High-Temperature Processing
- What is the principle of rotary kiln? Mastering Continuous Thermal Processing
- What equipment is used in pyrolysis? Choosing the Right Reactor for Your Feedstock and Products
- What are the different types of reactors in plastic pyrolysis? Choose the Right System for Your Waste
- What biomass is used in pyrolysis? Selecting the Optimal Feedstock for Your Goals



















