In essence, the synthesis of carbon nanotubes (CNTs) via Chemical Vapor Deposition (CVD) is a highly controlled process where a carbon-containing gas is thermally decomposed over a metallic catalyst. At high temperatures, the catalyst breaks down the gas, absorbs the carbon atoms, and then precipitates them in the form of a cylindrical nanotube structure. This method has become the industrial standard because it offers a superior balance of scalability, cost-effectiveness, and control compared to older techniques like laser ablation or arc discharge.
Chemical Vapor Deposition is the dominant commercial process for producing carbon nanotubes because it provides an unmatched ability to control the final product's structure at an industrial scale and a viable cost.

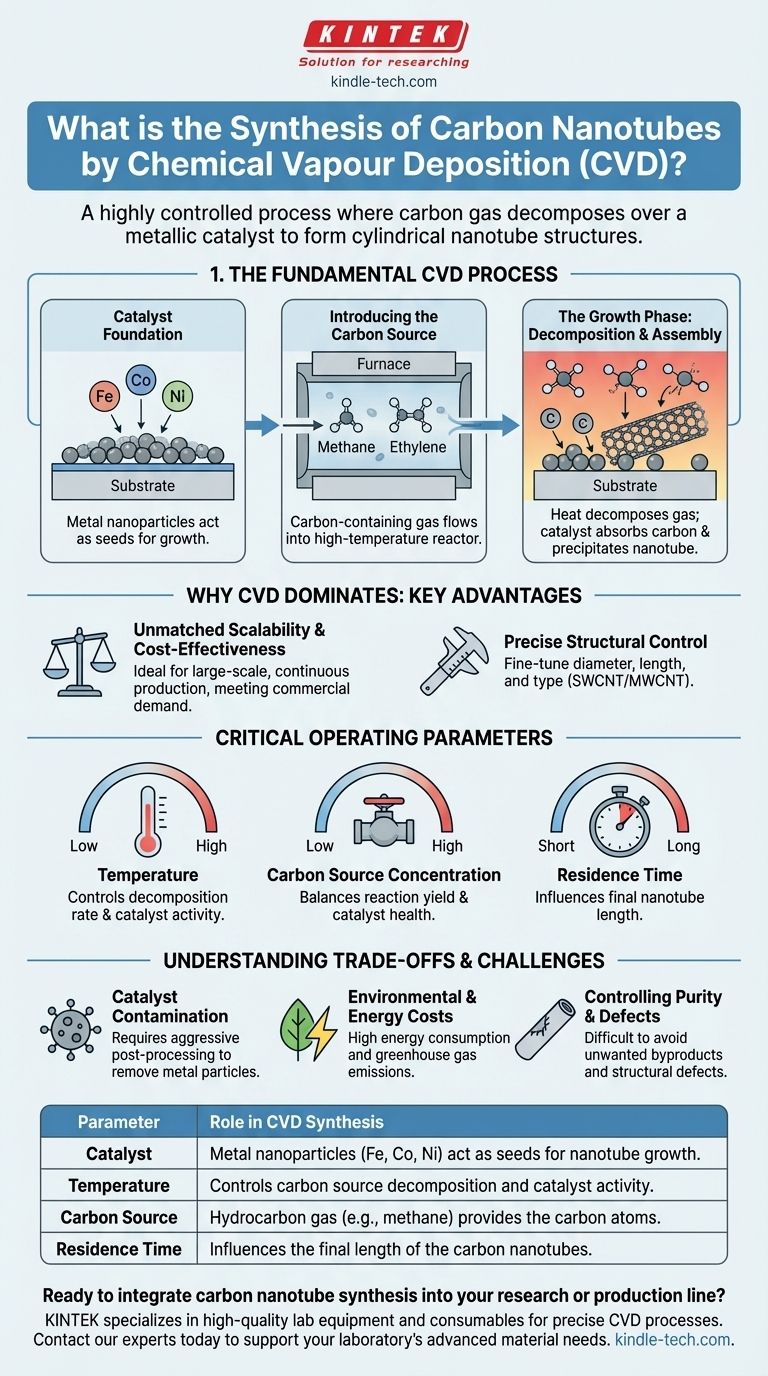
The Fundamental CVD Process: A Step-by-Step View
To truly understand CVD, it's best to visualize it as a precise, bottom-up assembly line happening at the nanoscale. The entire process hinges on the interaction between a catalyst, a carbon source, and heat.
The Catalyst Foundation
The process begins not with carbon, but with a catalyst. A substrate is prepared and coated with a thin layer of metallic nanoparticles, typically iron, cobalt, or nickel. These tiny metal islands serve as the "seeds" from which the nanotubes will grow.
Introducing the Carbon Source
The catalyst-coated substrate is placed inside a high-temperature furnace or reactor. A carefully controlled flow of a carbon-containing gas, such as methane, ethylene, or acetylene, is then introduced into the chamber.
The Growth Phase: Decomposition and Assembly
This is the core of the CVD reaction. The high temperature inside the reactor energizes the catalyst and causes the hydrocarbon gas to decompose, breaking its chemical bonds and releasing free carbon atoms.
The metallic catalyst particles absorb these carbon atoms. As the catalyst becomes supersaturated with carbon, it begins to precipitate the excess carbon out in a stable, structured form—a hollow tube. The nanotube continues to grow as long as the catalyst remains active and the carbon supply is available.
Why CVD Dominates: The Key Advantages
While other methods exist, catalytic CVD (often called CCVD) became the mainstream technique for several clear reasons that are critical for both research and industry.
Unmatched Scalability and Cost-Effectiveness
Compared to energy-intensive methods like arc discharge or laser ablation, CVD is far more suitable for large-scale, continuous production. This scalability makes it the most economically viable process for meeting commercial demand.
Precise Structural Control
CVD offers a remarkable degree of control over the final product. By carefully tuning the process parameters, operators can influence the nanotubes' diameter, length, and even whether they are single-walled (SWCNT) or multi-walled (MWCNT).
Mastering the Outcome: Critical Operating Parameters
The success and efficiency of the CVD process are directly governed by a few key operational variables. Understanding these allows for fine-tuning the synthesis to achieve desired results.
Temperature
Temperature is arguably the most critical parameter. It dictates the decomposition rate of the carbon source and the activity of the catalyst. An optimal temperature window is required; too low, and the reaction won't proceed efficiently, while too high can lead to the formation of undesirable amorphous carbon instead of clean nanotubes.
Carbon Source Concentration
The concentration of the hydrocarbon gas must be carefully balanced. Too low a concentration will starve the reaction and result in low yield, while too high a concentration can deactivate the catalyst or cause rapid, defective growth.
Residence Time
Residence time refers to how long the carbon-containing gas spends in the high-temperature reaction zone. This parameter directly influences the final length of the carbon nanotubes, with longer residence times generally producing longer tubes, up to a certain limit.
Understanding the Trade-offs and Challenges
Despite its advantages, the CVD process is not without its challenges. An objective assessment requires acknowledging its inherent limitations.
Catalyst Contamination
A significant drawback is that the final product is a composite of carbon nanotubes and the metallic catalyst particles used to grow them. Removing these impurities requires aggressive post-processing steps, often involving strong acids, which can damage the CNTs and create hazardous waste.
Environmental and Energy Costs
The high temperatures required for CVD demand significant energy consumption. Furthermore, the synthesis process itself is the primary contributor to the potential ecotoxicity of CNTs, releasing greenhouse gases that must be managed to limit the overall environmental impact.
Controlling Purity and Defects
Achieving a batch of perfectly uniform CNTs with zero defects is extremely difficult. The formation of unwanted byproducts, such as amorphous carbon, and structural defects in the nanotube walls remain persistent challenges that can compromise the material's ideal properties.
Making the Right Choice for Your Goal
Your approach to CVD should be guided by your end goal. The process is versatile, but optimizing for one outcome often means compromising on another.
- If your primary focus is large-scale industrial production: Catalytic CVD is the undisputed standard due to its unmatched cost-effectiveness and scalability for producing CNTs in bulk.
- If your primary focus is high-purity research samples: While CVD is a viable starting point, you must plan for intensive post-synthesis purification steps to remove catalyst residues and other impurities.
- If your primary focus is environmental sustainability: Investigate emerging CVD methods that leverage "green" or waste feedstocks, such as carbon dioxide or pyrolyzed methane, to reduce the life cycle impact.
By understanding these core principles and their practical trade-offs, you can effectively leverage the CVD process to achieve your specific material science or engineering objectives.
Summary Table:
| Parameter | Role in CVD Synthesis |
|---|---|
| Catalyst | Metal nanoparticles (Fe, Co, Ni) act as seeds for nanotube growth. |
| Temperature | Controls carbon source decomposition and catalyst activity. |
| Carbon Source | Hydrocarbon gas (e.g., methane) provides the carbon atoms. |
| Residence Time | Influences the final length of the carbon nanotubes. |
Ready to integrate carbon nanotube synthesis into your research or production line? KINTEK specializes in providing the high-quality lab equipment and consumables needed for precise CVD processes. Our expertise ensures you have the right tools for scalable, controlled synthesis. Contact our experts today to discuss how we can support your laboratory's advanced material needs.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- How high of temperature do carbon nanotubes in air have the ability to sustain? Understanding the Oxidation Limit
- What role does Chemical Vapor Deposition (CVD) equipment play in the preparation of C/C composites? Expert Analysis
- Why are carbon nanotubes important in industry? Unlocking Next-Generation Material Performance
- What is the floating catalyst method? A Guide to High-Yield CNT Production
- What are the methods of producing CNT? Scalable CVD vs. High-Purity Lab Techniques



















