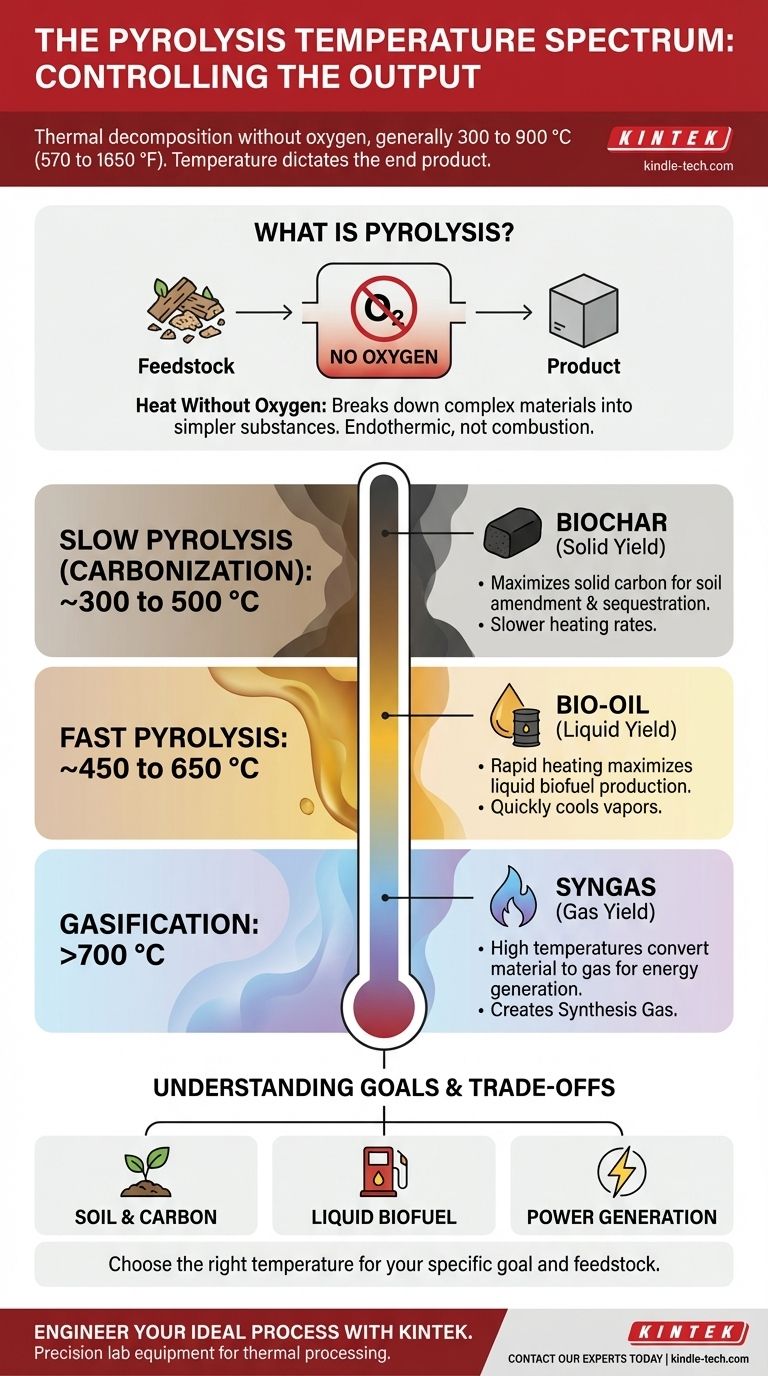
Pyrolysis is a thermal decomposition process that generally occurs in the temperature range of 300 to 900 °C (570 to 1650 °F). While the initial breakdown of organic materials like wood can begin at lower temperatures around 200–300 °C, the target temperature is dictated entirely by the desired end products.
The specific temperature used in pyrolysis is not a fixed number but a critical control parameter. The core insight is that changing the temperature directly changes the output, allowing you to choose between producing a solid (biochar), a liquid (bio-oil), or a gas (syngas).

What Exactly is Pyrolysis?
The Core Mechanism: Heat Without Oxygen
Pyrolysis is the chemical decomposition of materials at elevated temperatures in an anoxic environment, meaning in the absence of oxygen.
Without oxygen, the material cannot "burn" or combust in the traditional sense. Instead, the heat breaks down its complex chemical structures into simpler, often more valuable, substances.
Pyrolysis vs. Combustion
It is essential to distinguish pyrolysis from combustion (burning).
Combustion requires oxygen and is an exothermic reaction that releases energy as heat and light, producing primarily carbon dioxide, water, and ash.
Pyrolysis requires an oxygen-free environment and is an endothermic reaction, meaning it requires a continuous input of energy to sustain itself. It transforms a feedstock rather than just consuming it.
The Critical Role of Temperature
The temperature is the most significant factor influencing the final products of pyrolysis. Different temperature ranges are used to maximize the yield of one specific output.
Slow Pyrolysis (Carbonization): ~300 to 500 °C
This process uses lower temperatures and slower heating rates.
These conditions favor the production of a stable, carbon-rich solid known as biochar. The goal here is to maximize the solid yield.
Fast Pyrolysis: ~450 to 650 °C
This process uses moderate-to-high temperatures and very rapid heating.
These conditions are optimized to break down the material into vapors, which are then rapidly cooled and condensed to form a liquid product known as bio-oil or pyrolysis oil. This process maximizes liquid yield.
Gasification: >700 °C
At very high temperatures, the process is typically referred to as gasification.
The goal here is to break the material down almost completely into its gaseous components, creating a mixture called syngas (synthesis gas). This maximizes the gas yield for energy production.
Understanding the Trade-offs
Choosing a pyrolysis temperature involves balancing competing factors. There is no single "best" temperature; there is only the right temperature for a specific goal.
Temperature vs. Product Yield
The central trade-off is between the three product types. A temperature that maximizes biochar yield will inherently produce less bio-oil and syngas, and vice-versa. The process must be engineered specifically for the desired outcome.
Feedstock Variability
The ideal temperature range also depends on the feedstock (the starting material). Wood, agricultural waste, plastic, and tires all have different chemical compositions and will decompose differently, requiring fine-tuning of the process temperature.
Energy Input Costs
Reaching and maintaining higher temperatures requires a greater energy input. This increases the operational cost and complexity of the system. A key engineering challenge is to use some of the syngas produced during the process to provide the heat needed to sustain it.
Making the Right Choice for Your Goal
Your target temperature is defined by the product you want to create.
- If your primary focus is soil amendment or carbon sequestration: Target a lower-temperature slow pyrolysis (around 400 °C) to maximize the production of stable biochar.
- If your primary focus is creating a liquid biofuel: Target a mid-range fast pyrolysis (around 500 °C) with rapid heating to maximize the yield of bio-oil.
- If your primary focus is generating fuel gas for power: Target a higher-temperature process like gasification (>700 °C) to convert the feedstock primarily into syngas.
By understanding the relationship between temperature and outputs, you can control the pyrolysis process to achieve your specific chemical or energy goals.
Summary Table:
| Target Product | Process Type | Typical Temperature Range | Key Characteristic |
|---|---|---|---|
| Biochar | Slow Pyrolysis | 300–500 °C | Maximizes solid carbon yield for soil/sequestration |
| Bio-oil | Fast Pyrolysis | 450–650 °C | Rapid heating maximizes liquid biofuel production |
| Syngas | Gasification | >700 °C | High temperatures maximize gas yield for energy |
Ready to engineer your pyrolysis process for optimal results?
At KINTEK, we specialize in precision lab equipment and consumables for thermal processing. Whether you're developing biochar for carbon sequestration, optimizing bio-oil production, or generating syngas for energy, our solutions provide the exact temperature control and reliability you need.
Let's build your ideal pyrolysis setup together. Contact our experts today to discuss your specific feedstock and target products!
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory High Pressure Vacuum Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What is the ceramic tube high temperature? From 1100°C to 1800°C, Choose the Right Material
- What are the advantages of a tube furnace? Achieve Superior Temperature Uniformity and Control
- What are the advantages of a tube furnace? Achieve Superior Thermal Control and Purity
- What are the applications of tube furnace? Unlock Precise High-Temperature Processing
- What are the benefits of a tube furnace? Achieve Superior Temperature & Atmosphere Control



















