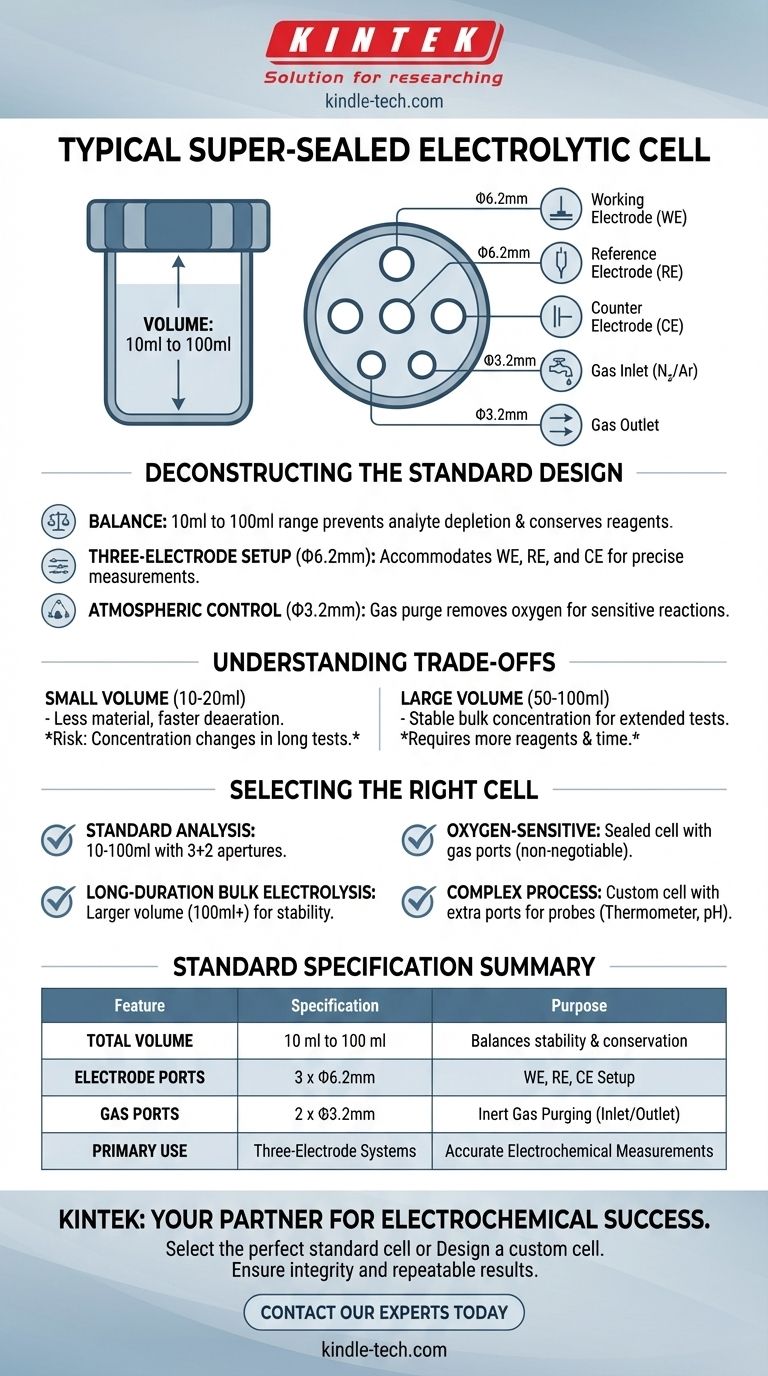
To be precise, a typical super-sealed electrolytic cell used in a three-electrode system has a volume ranging from 10ml to 100ml. Its standard aperture configuration consists of five holes: three with a diameter of Φ6.2mm for the electrodes and two smaller holes of Φ3.2mm for gas management.
The standard design of a super-sealed cell is intentionally practical, providing the necessary ports for a three-electrode setup while enabling strict atmospheric control. Understanding the function of each aperture is the key to choosing the correct cell for your specific electrochemical experiment.
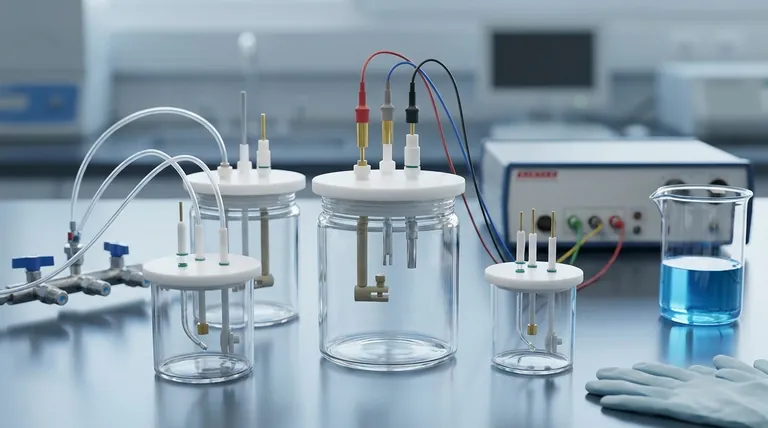
Deconstructing the Standard Cell Design
The specifications of an electrolytic cell are not arbitrary; they are designed to support reproducible and accurate electrochemical measurements. The volume and aperture layout directly impact factors like reactant concentration, atmospheric purity, and experimental setup.
The Purpose of the Volume Range
The common 10ml to 100ml volume represents a critical balance. This range is large enough to prevent rapid depletion of the analyte during an experiment, which would alter the bulk concentration and skew results.
At the same time, it is small enough to conserve often-expensive solvents, supporting electrolytes, and active materials. While other cell types can range up to 1000ml or more, the super-sealed design is optimized for typical laboratory-scale analysis.
The Three-Electrode Apertures (Φ6.2mm)
The three larger Φ6.2mm ports are the core of the experimental setup. They are specifically sized to accommodate the standard electrodes in a three-electrode system:
- Working Electrode (WE): Where the reaction of interest occurs.
- Reference Electrode (RE): Provides a stable potential against which the working electrode's potential is measured.
- Counter Electrode (CE): Completes the electrical circuit, passing a current equal and opposite to that of the working electrode.
The Gas Inlet and Outlet Apertures (Φ3.2mm)
The two smaller Φ3.2mm ports are what define the cell as "sealed" and are critical for controlling the atmosphere inside.
Their primary function is to allow for purging the electrolyte with an inert gas, such as nitrogen or argon. This process removes dissolved oxygen, which can interfere with many electrochemical reactions. One port serves as the gas inlet, while the other acts as the outlet.
Understanding the Trade-offs
The standard configuration is a versatile starting point, but it's not universally perfect. Deviating from the standard involves trade-offs that you must consider based on your experimental goals.
Volume and Experimental Duration
A smaller volume cell (~10-20ml) requires less material and can be deaerated with gas more quickly. However, it is more susceptible to concentration changes during long-running experiments like bulk electrolysis.
A larger volume cell (~50-100ml) provides a more stable bulk concentration for extended tests but requires more reagents and longer purging times.
Sealed vs. Non-Sealed Designs
A super-sealed cell with five apertures is essential for any experiment sensitive to atmospheric gases.
For simple experiments where oxygen interference is not a concern, a basic, non-sealed cell with just three holes for the electrodes may be sufficient and more accessible.
The Need for Customization
The standard five-hole setup does not account for additional instrumentation. If your experiment requires simultaneous monitoring of other parameters, you will need a custom configuration. Common additions include ports for a thermometer, a pH probe, or a sampling syringe.
Selecting the Right Cell for Your Experiment
Your choice should be dictated entirely by the demands of your specific analysis.
- If your primary focus is standard analysis (e.g., cyclic voltammetry): The standard 10ml to 100ml cell with the 3+2 aperture configuration is almost always the correct choice.
- If your primary focus is studying oxygen-sensitive reactions: A sealed cell with its gas inlet/outlet ports is non-negotiable for deaerating the solution.
- If your primary focus is long-duration bulk electrolysis: Opt for a larger volume cell (100ml or more) to ensure the stability of your analyte concentration.
- If your primary focus is a complex process requiring extra monitoring: You must request a custom cell with additional apertures for your specific probes.
By understanding the function behind the standard design, you can confidently select the right electrolytic cell to ensure the integrity and success of your research.
Summary Table:
| Feature | Standard Specification | Purpose |
|---|---|---|
| Total Volume | 10 ml to 100 ml | Balances analyte stability with reagent conservation |
| Electrode Ports | 3 x Φ6.2mm holes | Accommodates working, reference, and counter electrodes |
| Gas Ports | 2 x Φ3.2mm holes | For inert gas purging (inlet/outlet) to remove oxygen |
| Primary Use | Three-electrode systems | Ensures accurate and reproducible electrochemical measurements |
Ensure the integrity of your electrochemical research with the right equipment.
The standard 10ml-100ml, five-aperture cell is a versatile starting point, but your specific experiment may demand a custom solution. At KINTEK, we specialize in lab equipment and consumables, providing reliable electrolytic cells and expert support for your laboratory needs.
We can help you:
- Select the perfect standard cell for techniques like cyclic voltammetry.
- Design a custom cell with additional ports for probes (e.g., thermometer, pH) or larger volumes for bulk electrolysis.
Let us provide the precise tools you need for successful, repeatable results.
Contact our experts today to discuss your electrochemical cell requirements!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup
- How should the five-port water bath electrolytic cell be operated during an experiment? Master Precise Control for Reliable Results
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan



















