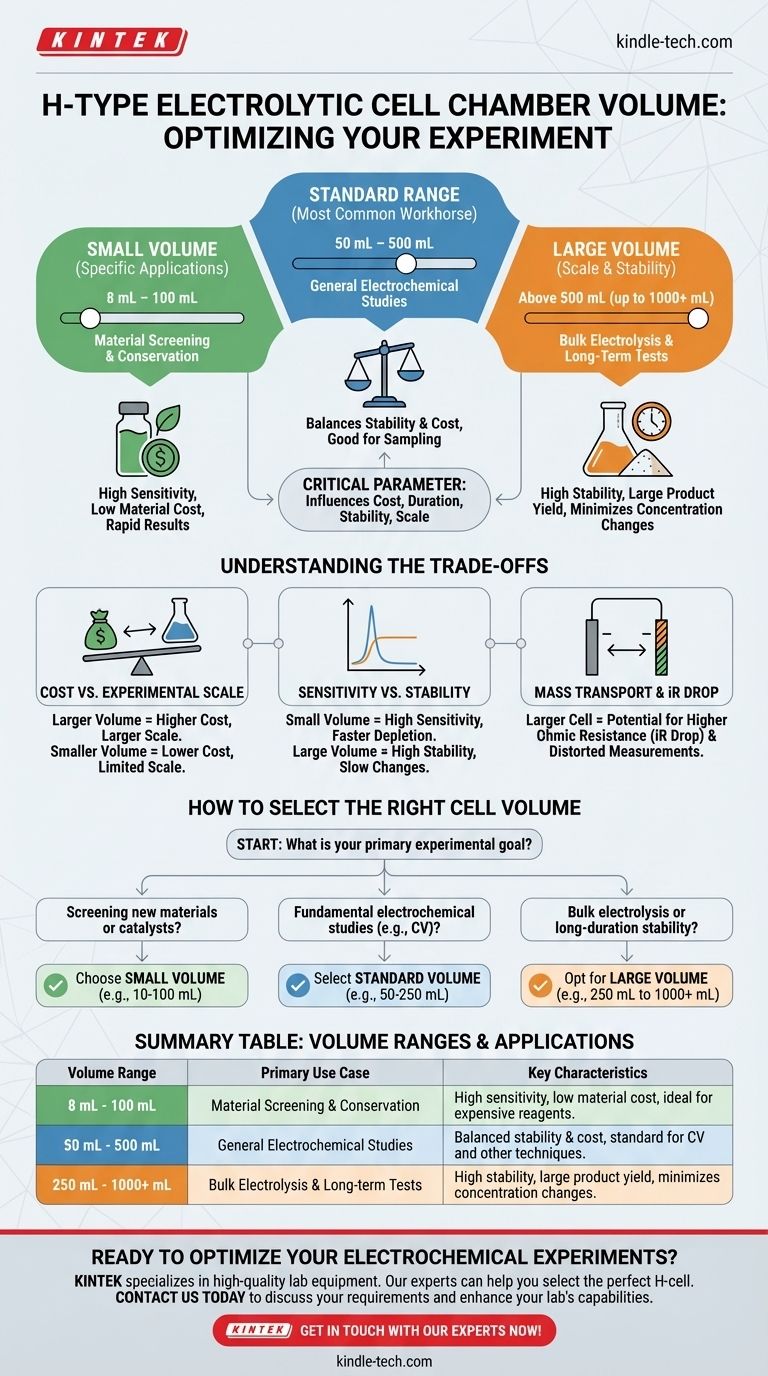
The typical volume for a single chamber in a standard H-type electrolytic cell generally ranges from 30 mL to 500 mL. While this is the most common workhorse range, specific applications have driven the development of cells with volumes as small as 8 mL for material conservation and as large as 1000 mL (1 L) or more for bulk electrolysis.
The specific volume you choose is not arbitrary; it is a critical experimental parameter. The central decision revolves around balancing the cost of your electrolyte and reactants against the required duration, stability, and scale of your electrochemical experiment.

Why Chamber Volume is a Critical Parameter
An H-type cell's defining feature is the separation of the anode and cathode chambers, typically by an ion-exchange membrane or a glass frit. The volume of these chambers is a fundamental design choice that directly influences your results.
The Standard Range: 50 mL to 500 mL
This range is the most common for general-purpose electrochemistry. It represents a practical balance for many standard three-electrode experiments.
A volume in this range is large enough to allow for repeated sampling without significantly altering the bulk electrolyte concentration. It also provides sufficient space for proper electrode placement, which is crucial for accurate measurements.
Small-Volume Cells: Below 100 mL
Cells with smaller volumes, often from 8 mL to 100 mL, are designed for specific, high-value applications.
Their primary advantage is conservation of materials. When working with expensive or rare electrolytes, catalysts, or substrates, a small-volume cell minimizes waste and reduces experimental cost. They are ideal for initial material screening and characterization studies.
Large-Volume Cells: Above 500 mL
Large-volume cells are used when the goal is scale or long-term stability.
These are necessary for bulk electrolysis, where the objective is to produce a significant quantity of a product. They are also used in long-duration experiments, such as corrosion testing or battery cycling, where a large reservoir of electrolyte is needed to minimize concentration changes over time.
Understanding the Trade-offs
Choosing a cell volume involves a series of trade-offs. Understanding these compromises is key to designing a valid experiment.
Cost vs. Experimental Scale
This is the most straightforward trade-off. A larger volume allows for larger-scale synthesis or longer experiments but requires a proportionally larger investment in chemicals.
A smaller volume dramatically cuts material costs but limits the amount of product you can generate or the duration of your test.
Sensitivity vs. Stability
A small-volume cell is highly sensitive. The concentration of a generated species will rise quickly, which can be ideal for analytical detection. However, this also means the reactants are depleted faster, potentially shortening the valid experimental window.
A large-volume cell provides greater stability. The bulk concentration changes very slowly, making it easier to maintain steady-state conditions for long periods.
Mass Transport and iR Drop
Volume is intrinsically linked to the cell's geometry. In a larger cell, the distance between the electrodes and the separating membrane can be greater, potentially increasing the ohmic resistance (iR drop) of the solution.
This can distort electrochemical measurements, especially at high currents. Careful placement of the reference electrode using a Luggin capillary becomes even more critical in larger cells to mitigate this effect.
How to Select the Right Cell Volume
Your experimental goal should be the primary driver of your decision. Consider the following guidelines to make an informed choice.
- If your primary focus is screening new materials or catalysts: Choose a small-volume cell (e.g., 10-100 mL) to conserve expensive materials and get rapid results.
- If your primary focus is fundamental electrochemical studies (e.g., CV, chronoamperometry): Select a standard, mid-range volume (e.g., 50-250 mL) that offers a good balance of stability and reasonable material cost.
- If your primary focus is bulk electrolysis or long-duration stability testing: Opt for a large-volume cell (e.g., 250 mL to 1000+ mL) to ensure stable conditions and produce sufficient product.
By matching the cell volume to your specific objective, you ensure the validity of your data and the efficiency of your resources.
Summary Table:
| Volume Range | Primary Use Case | Key Characteristics |
|---|---|---|
| 8 mL - 100 mL | Material Screening & Conservation | High sensitivity, low material cost, ideal for expensive reagents. |
| 50 mL - 500 mL | General Electrochemical Studies | Balanced stability & cost, standard for CV and other techniques. |
| 250 mL - 1000+ mL | Bulk Electrolysis & Long-term Tests | High stability, large product yield, minimizes concentration changes. |
Ready to optimize your electrochemical experiments?
Choosing the right H-type electrolytic cell is critical for achieving accurate and reproducible results. KINTEK specializes in high-quality lab equipment, including a range of H-cells designed for various volumes and applications—from material screening to bulk synthesis.
Our experts can help you select the perfect cell to match your specific needs, ensuring you balance cost, scale, and performance effectively. Contact us today to discuss your requirements and enhance your lab's capabilities.
Get in touch with our experts now!
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What experimental conditions need to be controlled when using an H-type electrolytic cell? Ensure Reliable and Repeatable Results
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results



















