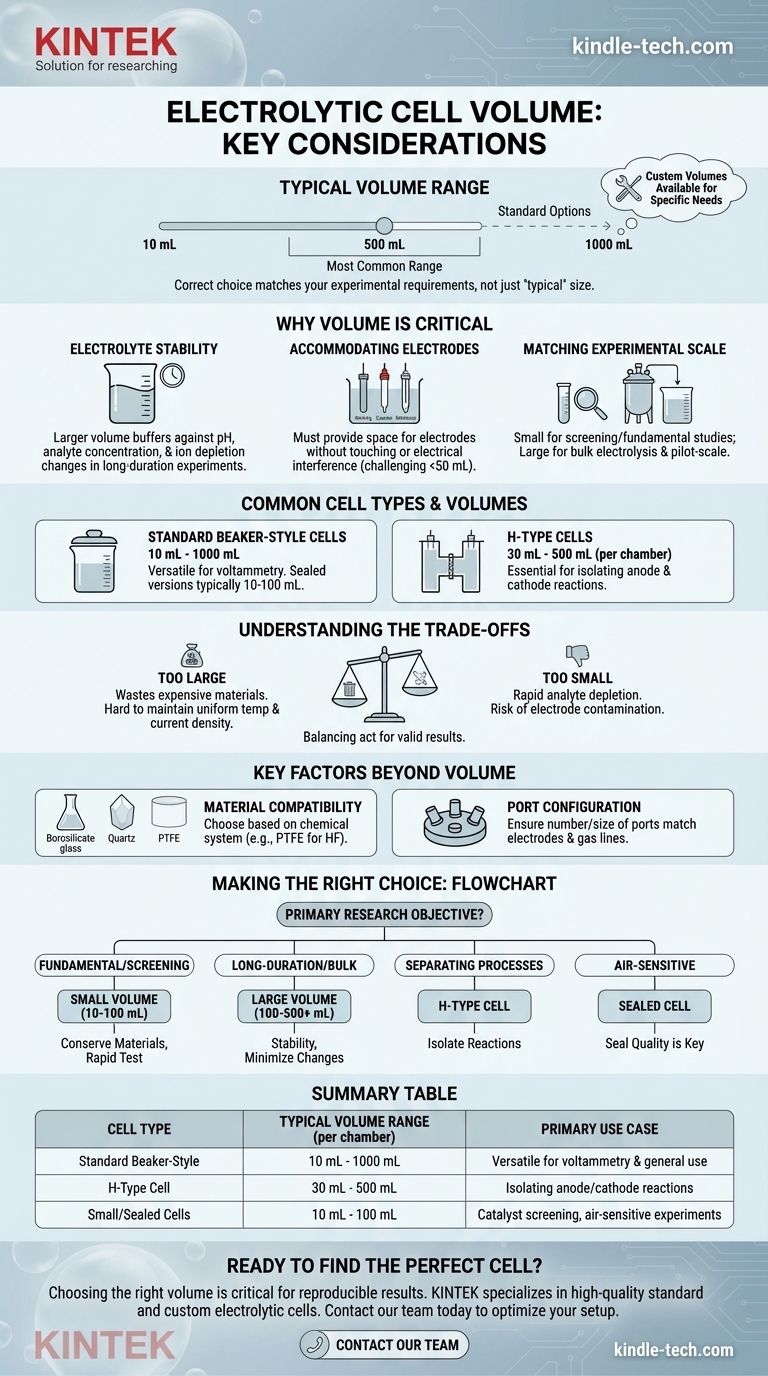
Typically, the volume for a single chamber of an electrolytic cell ranges from as small as 10 mL to as large as 500 mL, with some standard options extending up to 1000 mL. This range covers the vast majority of laboratory applications, from fundamental research to small-scale synthesis. Crucially, most suppliers also offer custom fabrication for volumes outside this common range to meet specific experimental needs.
While a standard electrolytic cell chamber volume is typically between 50 mL and 500 mL, the correct choice is not about finding a "typical" size. It's about matching the cell volume to your specific experimental requirements, such as the scale of your reaction, the size of your electrodes, and the need for electrolyte stability.

Why Volume is a Critical Parameter
Choosing the right cell volume is one of the first and most important decisions in designing an electrochemical experiment. It directly influences the validity, reproducibility, and scalability of your results.
Impact on Electrolyte Stability
A larger volume of electrolyte acts as a buffer against change. In long-duration experiments, it minimizes shifts in pH, analyte concentration, and ion depletion near the electrodes.
This stability is critical for ensuring that the conditions of your experiment remain constant, leading to more reliable and interpretable data.
Accommodating Electrodes and Probes
The cell must provide enough space to house the working electrode, counter electrode, and reference electrode without them touching or causing electrical interference.
Smaller volumes (under 50 mL) can present a physical challenge, requiring careful placement to avoid short-circuiting or distorted electrochemical signals.
Matching the Experimental Scale
The required volume is fundamentally tied to your goal. Small-volume cells are ideal for screening catalysts or performing fundamental studies with expensive materials.
Larger volumes are necessary for bulk electrolysis, where the goal is to produce a significant quantity of product, or for pilot-scale process development.
Common Cell Types and Their Volumes
Different experimental setups require different cell geometries, each with a common volume range.
Standard Beaker-Style Cells (10 mL - 1000 mL)
These are the most common type, typically made of glass or PTFE, and used for standard three-electrode voltammetry. Their wide volume range makes them versatile for many applications.
Super-sealed versions of these cells, designed for air-sensitive or gas-tight experiments, are often found in the smaller 10 mL to 100 mL range to ensure a reliable seal.
H-Type Cells (30 mL - 500 mL per chamber)
H-type cells consist of two chambers separated by a membrane or frit. They are essential for experiments where you must isolate the products or processes occurring at the anode and cathode.
The volume range, typically 30 mL to 500 mL, applies to each individual chamber.
Understanding the Trade-offs
Choosing a volume is a balancing act. Optimizing for one factor can introduce limitations in another.
The Problem with Excessively Large Volumes
Using a cell that is too large wastes expensive materials, such as the electrolyte, solvent, and any dissolved catalysts or analytes.
It can also make it difficult to achieve a uniform temperature and may require larger, more costly electrodes to maintain appropriate current densities.
The Risks of Excessively Small Volumes
A volume that is too small can lead to rapid depletion of the analyte, causing significant concentration changes during the experiment and skewing results.
The proximity of the electrodes can also cause contamination, where products from the counter electrode diffuse to and react at the working electrode.
Key Factors Beyond Volume
Beyond the raw milliliter number, you must consider other critical design features that are often linked to cell size.
Material Compatibility
Cells are commonly made of borosilicate glass, quartz, or PTFE. The choice depends entirely on your chemical system—for instance, PTFE is required for experiments involving hydrofluoric acid, which etches glass.
Port Configuration
Standard cells often come with a lid featuring a specific number and size of ports. A common configuration includes three larger ports (~6.2 mm) for the electrodes and two smaller ports (~3.2 mm) for gas sparging or venting. Ensure the ports match your equipment.
Making the Right Choice for Your Experiment
To select the appropriate cell, align the volume and type with your primary research objective.
- If your primary focus is fundamental research or catalyst screening: A smaller volume (10-100 mL) is efficient for conserving precious materials and allows for rapid testing.
- If your primary focus is long-duration experiments or bulk synthesis: A larger volume (100-500+ mL) provides greater stability by minimizing changes in electrolyte concentration and temperature.
- If your primary focus is separating anodic and cathodic processes: An H-type cell is non-negotiable, and the chamber volume will depend on the scale of your separated reactions.
- If your primary focus is air-sensitive or gas-related electrochemistry: A sealed cell is necessary, where the quality of the seal is often more critical than the specific volume.
Ultimately, selecting the correct cell volume is about ensuring the integrity and relevance of your electrochemical data.
Summary Table:
| Cell Type | Typical Volume Range (per chamber) | Primary Use Case |
|---|---|---|
| Standard Beaker-Style | 10 mL - 1000 mL | Versatile for voltammetry and general use |
| H-Type Cell | 30 mL - 500 mL | Isolating anode/cathode reactions |
| Small/Sealed Cells | 10 mL - 100 mL | Catalyst screening, air-sensitive experiments |
Ready to find the perfect electrolytic cell for your research?
Choosing the right volume is critical for valid, reproducible results. KINTEK specializes in high-quality lab equipment, including a wide range of standard and custom electrolytic cells for all your laboratory needs. Our experts can help you select the ideal cell to match your experimental scale, material compatibility, and performance requirements.
Contact our team today to discuss your specific application and ensure your electrochemical setup is optimized for success.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What is the significance of EIS testing for composite catalysts? Optimize Charge Transfer with Precision Workstations
- How should faults with the electrolytic cell be handled? A Guide to Safe Diagnosis and Repair
- What advantages do split cells and ion-exchange membranes offer in gold electrowinning? Boost Efficiency & Purity
- What are the primary functions of the diaphragm within a seawater electrolysis cell? Enhance Safety and Efficiency
- What is the electrolytic cell? A Guide to Forcing Chemical Reactions with Electricity
- What is a flat cell for corrosion testing? Achieve Non-Destructive, In-Situ Analysis
- What functions do electrolytic cells perform in PEC water splitting? Optimize Your Photoelectrochemical Research
- Why is a precise high-temperature heating and control system necessary for nitrate-to-ammonia electrosynthesis reactors?



















