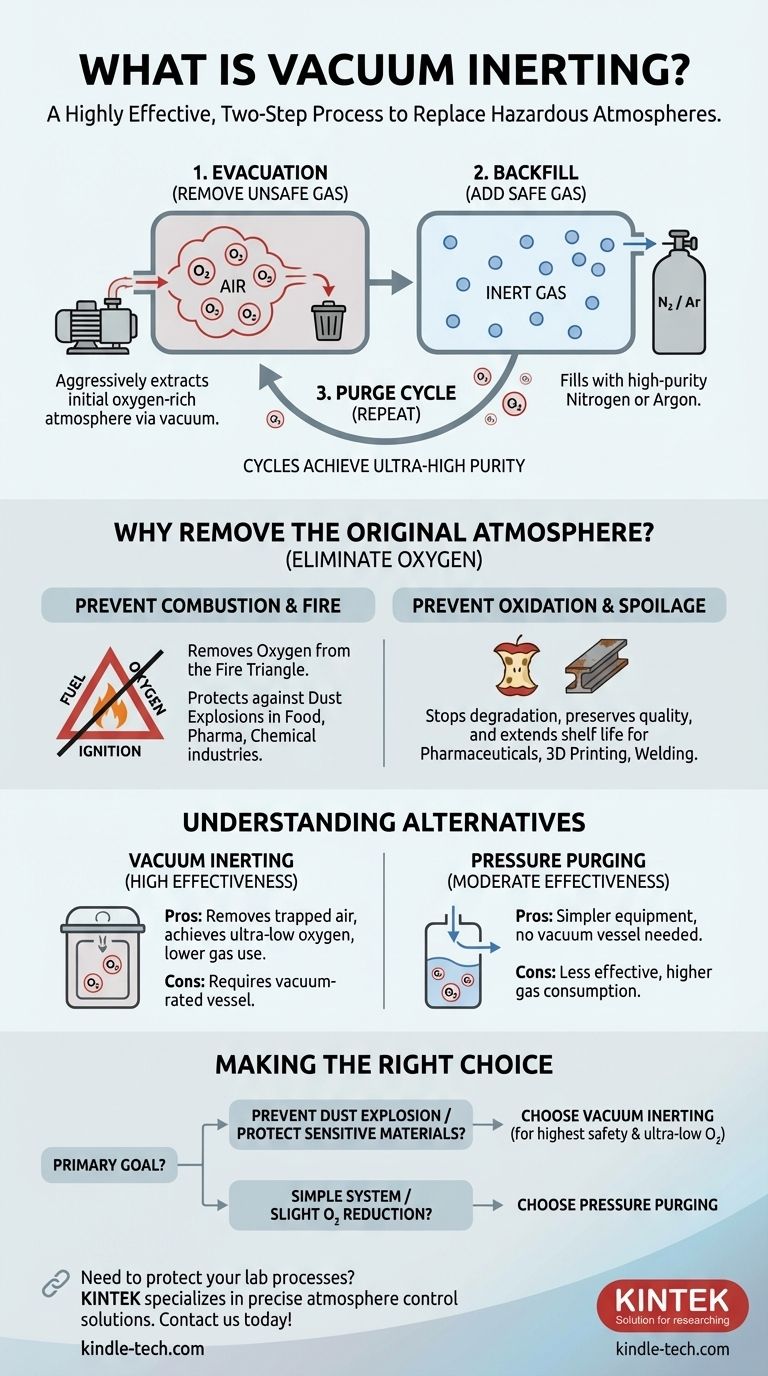
In simple terms, vacuum inerting is a highly effective, two-step method for replacing a hazardous or reactive atmosphere inside a sealed container with a safe, non-reactive one. The process first uses a vacuum pump to remove the original atmosphere (like oxygen-rich air), and then breaks that vacuum by backfilling the container with an inert gas, such as nitrogen or argon. This is often repeated in cycles to achieve exceptionally high purity.
The core purpose of vacuum inerting is not just to add a safe gas, but to first aggressively remove an unsafe one. By pulling a vacuum, you ensure that the unwanted atmosphere is physically extracted rather than just diluted, making it one of the most reliable methods for preventing explosions and protecting sensitive materials.

Why Removing the Original Atmosphere is Critical
The decision to use vacuum inerting stems from the need to eliminate the risks posed by a container's default atmosphere, which is usually ambient air. Air is approximately 21% oxygen, a highly reactive gas that creates two primary problems: combustion and oxidation.
The Threat of Combustion and Fire
Any process involving fine, combustible powders—such as those in the food processing, pharmaceutical, or chemical industries—carries the risk of a dust explosion.
For a fire or explosion to occur, three elements are needed: fuel (the dust), an ignition source (like a spark or hot surface), and oxygen. Vacuum inerting systematically removes the oxygen component of this "fire triangle," rendering the mixture non-flammable.
The Problem of Oxidation and Spoilage
Many materials are sensitive to oxygen and moisture. These reactions can degrade product quality, reduce shelf life, or create unwanted chemical byproducts.
For example, certain pharmaceuticals lose their efficacy, food products spoil, and metals can form undesirable oxide layers during high-temperature manufacturing processes like 3D printing or welding. By replacing oxygen with an inert gas, product integrity is preserved.
The Vacuum Inerting Process Explained
The effectiveness of vacuum inerting lies in its cyclical nature. Each cycle drastically reduces the concentration of the contaminant gas, achieving purity levels that are difficult to reach with other methods.
Step 1: The Evacuation
First, a vacuum pump is connected to the sealed vessel. The pump removes the air and any other gases, lowering the internal pressure.
This step is the most critical differentiator. It physically removes the majority of the oxygen molecules, rather than just diluting them.
Step 2: The Backfill
Once the target vacuum level is reached, the vacuum pump is isolated, and a valve is opened to introduce a high-purity inert gas, typically nitrogen or argon.
The gas flows into the vessel until the pressure returns to atmospheric pressure or a desired positive pressure.
Step 3: The Purge Cycle
For applications requiring extremely low oxygen levels, this process is repeated. Each "purge cycle" further reduces the remaining oxygen concentration exponentially.
For instance, pulling a vacuum that removes 90% of the air reduces oxygen to about 2.1%. A second cycle would reduce that remaining oxygen by another 90%, leaving only 0.21%, and so on.
Understanding the Alternatives and Trade-offs
Vacuum inerting is powerful, but it's not the only method available. Its primary alternative is pressure-hold or flow-through purging.
Vacuum Inerting vs. Pressure Purging
Pressure purging involves continuously flowing an inert gas into a vessel and letting it exit through a vent. This displaces the oxygen through dilution.
This method is simpler and doesn't require a vacuum-rated vessel, but it uses significantly more inert gas and is less effective at removing trapped pockets of air in complex geometries or fine powders.
Choosing the Right Inert Gas
Nitrogen is the most common choice for inerting because it is effective, inexpensive, and widely available.
Argon is used in more specialized, high-temperature applications like welding exotic metals. It is denser than nitrogen and even less reactive, but it comes at a higher cost.
Key Equipment and Safety Considerations
The most important requirement for vacuum inerting is that the vessel must be rated to withstand a full vacuum without collapsing. This adds to the cost and complexity of the system.
Furthermore, inert gases are asphyxiants. Proper ventilation and oxygen monitoring are critical safety measures in any area where inert gas is used, as a leak can displace breathable air in a confined space.
Making the Right Choice for Your Goal
Selecting the correct inerting strategy depends entirely on the sensitivity of your process, the design of your equipment, and your safety requirements.
- If your primary focus is preventing a dust explosion: Vacuum inerting provides the highest level of safety by thoroughly removing oxygen, especially from dense powders.
- If your primary focus is protecting highly sensitive materials: The repeatable purge cycles of vacuum inerting allow you to achieve the ultra-low oxygen levels needed for pharmaceuticals, electronics, or specialty chemicals.
- If you are working with a simple, open system or only need to reduce oxygen slightly: A continuous flow-through purge with nitrogen may be a more cost-effective solution.
- If your vessel cannot withstand a vacuum: You must use an alternative like pressure purging and accept that it may be less efficient and consume more gas.
Ultimately, vacuum inerting is the definitive choice when the complete and verified removal of a reactive atmosphere is non-negotiable.
Summary Table:
| Aspect | Vacuum Inerting | Pressure Purging |
|---|---|---|
| Principle | Removes oxygen via vacuum cycles | Dilutes oxygen with continuous gas flow |
| Effectiveness | High (removes trapped air) | Moderate (less effective in complex geometries) |
| Gas Consumption | Lower | Higher |
| Equipment Needs | Vacuum-rated vessel required | Standard vessel sufficient |
| Best For | Ultra-low oxygen levels, sensitive materials, dense powders | Simple systems, slight oxygen reduction |
Need to protect your lab processes from oxygen-related risks? KINTEK specializes in lab equipment and consumables for precise atmosphere control. Our experts can help you select the right vacuum inerting or purging solution to ensure safety, preserve material integrity, and enhance your lab's efficiency. Contact us today for a consultation tailored to your laboratory needs!
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What is an inert condition? A Guide to Preventing Fires and Explosions
- Why nitrogen is used in furnace? A Cost-Effective Shield for High-Temperature Processes
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab
- What is the purpose of inert atmosphere? A Guide to Protecting Your Materials and Processes
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety



















