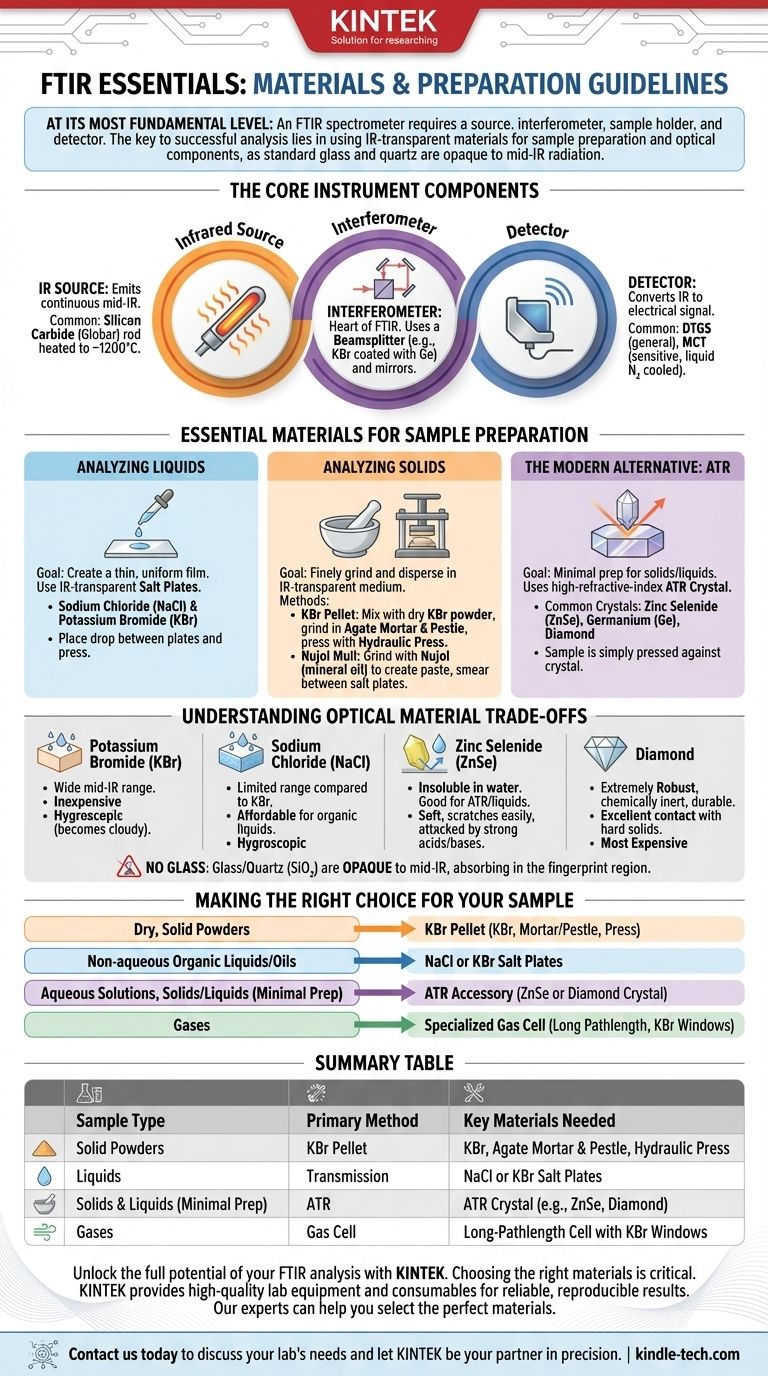
At its most fundamental level, an FTIR spectrometer requires a source of infrared radiation, an interferometer (containing a beamsplitter and mirrors), a sample holder, and a detector. However, the most critical "materials" you will interact with are those used for sample preparation and the optical components, as they must be transparent to infrared light.
The core principle to understand is that standard materials like glass and quartz are opaque to mid-infrared radiation. Therefore, the specific materials needed for FTIR—from sample windows to accessories—are dictated entirely by the physical state of your sample and the spectral range you wish to analyze.

The Core Instrument Components
Before discussing sample handling, it's essential to understand the materials inside the instrument itself. These are the non-negotiable components that make the technique possible.
The Infrared Source
This is a material that, when heated, emits a continuous spectrum of mid-infrared radiation. A common source is a silicon carbide rod heated to about 1200°C, often called a Globar.
The Interferometer
This is the heart of the FTIR. It uses a beamsplitter, typically made of a material like potassium bromide (KBr) coated with germanium, to split the IR beam. The beams are then reflected off mirrors before being recombined.
The Detector
The detector converts the infrared light signal into an electrical signal. Common detector materials include deuterated triglycine sulfate (DTGS) for general-purpose use and the more sensitive mercury cadmium telluride (MCT), which must be cooled with liquid nitrogen.
Essential Materials for Sample Preparation
This is where your choices directly impact the quality of your results. The materials you need depend entirely on whether your sample is a solid, liquid, or gas.
Analyzing Liquid Samples
For liquids, the goal is to create a very thin, uniform film for the IR beam to pass through.
This is typically done using "salt plates," which are polished, flat windows made of IR-transparent materials. You place a drop of the liquid between two plates and gently press them together. The most common materials are sodium chloride (NaCl) and potassium bromide (KBr).
Analyzing Solid Samples
Solids must be finely ground and dispersed in an IR-transparent medium to avoid scattering the IR beam.
The most common method is the KBr pellet. Here, you mix a tiny amount of your solid sample with dry potassium bromide powder. The mixture is ground to a fine powder in an agate mortar and pestle, then pressed into a thin, transparent pellet using a hydraulic press.
An older method is the Nujol mull. A small amount of the solid is ground with a drop of Nujol (mineral oil) to create a paste, which is then smeared between two salt plates.
The Modern Alternative: ATR
Attenuated Total Reflectance (ATR) is a popular, modern technique that requires minimal sample prep for both solids and liquids. The key material is the ATR crystal, which is a high-refractive-index material.
Common ATR crystal materials include zinc selenide (ZnSe), germanium (Ge), and diamond. The sample is simply pressed firmly against the crystal for analysis.
Understanding the Trade-offs of Optical Materials
The windows and plates you choose are critical. Using the wrong material will result in a poor or nonexistent spectrum.
Why You Can't Use Glass
Glass and quartz are made of silicon dioxide (SiO₂). The Si-O bonds strongly absorb most of the mid-infrared radiation, making them completely opaque in the fingerprint region of an IR spectrum.
Common IR-Transparent Materials
- Potassium Bromide (KBr): This is the workhorse for transmission FTIR. It's inexpensive and transparent over a very wide mid-IR range. Its major drawback is that it is hygroscopic, meaning it readily absorbs moisture from the air and will become cloudy.
- Sodium Chloride (NaCl): Similar to KBr, NaCl is also hygroscopic. It has a slightly more limited range than KBr but is a very common and affordable choice for analyzing organic liquids.
- Zinc Selenide (ZnSe): This material is a popular choice for ATR and liquid cells because it is insoluble in water. However, it is soft, scratches easily, and is attacked by strong acids and bases.
- Diamond: Used almost exclusively in ATR accessories, diamond is extremely robust, chemically inert, and durable. Its hardness allows for excellent contact with hard solid samples, but it is the most expensive option.
Making the Right Choice for Your Sample
Your sample's properties should be the sole guide for selecting the correct materials and methods for your analysis.
- If your primary focus is analyzing dry, solid powders: The KBr pellet method is the classic, low-cost approach, requiring KBr powder, a mortar and pestle, and a pellet press.
- If your primary focus is analyzing non-aqueous organic liquids or oils: Standard NaCl or KBr salt plates are the most direct and economical materials.
- If your primary focus is analyzing aqueous solutions or a wide variety of solids and liquids with minimal prep: An ATR accessory with a versatile ZnSe or a highly durable diamond crystal is the most efficient choice.
- If your primary focus is analyzing gases: You will need a specialized gas cell with IR-transparent windows (often KBr) designed to provide a long pathlength for the IR beam.
Choosing the right materials transforms FTIR from a complex instrument into a powerful and accessible analytical tool.
Summary Table:
| Sample Type | Primary Method | Key Materials Needed |
|---|---|---|
| Solid Powders | KBr Pellet | Potassium Bromide (KBr), Agate Mortar & Pestle, Hydraulic Press |
| Liquids | Transmission | Sodium Chloride (NaCl) or KBr Salt Plates |
| Solids & Liquids (Minimal Prep) | ATR | ATR Crystal (e.g., ZnSe, Diamond) |
| Gases | Gas Cell | Long-Pathlength Cell with KBr Windows |
Unlock the full potential of your FTIR analysis with KINTEK.
Choosing the right materials is critical for success. Whether you're preparing KBr pellets, analyzing liquids with salt plates, or leveraging the speed of ATR, KINTEK provides the high-quality lab equipment and consumables you need for reliable, reproducible results.
Our experts can help you select the perfect materials for your specific application, ensuring your FTIR delivers accurate data every time.
Contact us today to discuss your lab's needs and let KINTEK be your partner in precision: Get in Touch
Visual Guide
Related Products
- High Purity Zinc Foil for Battery Lab Applications
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
- Automatic Laboratory Heat Press Machine
People Also Ask
- How thick is sputtering gold? Achieve Angstrom-Level Precision for Your Application
- How much is the cost of pyrolysis plant? A Guide to Budgeting for Your Specific Project
- What are the process advantages of vacuum pump suction filtration for hydrophobic carriers? Enhance Your Bioprocessing
- How is thin film thickness measured? Achieve Atomic-Level Precision for Your Films
- What is pyrolysis oil used for? Unlocking Renewable Energy from Waste
- What is a lab furnace used for? Transform Materials with Precise Thermal Control
- What is heat treatment in manufacturing process? Transform Material Properties for Superior Performance
- What is the problem with plastic pyrolysis? Key Challenges and Environmental Trade-offs



















