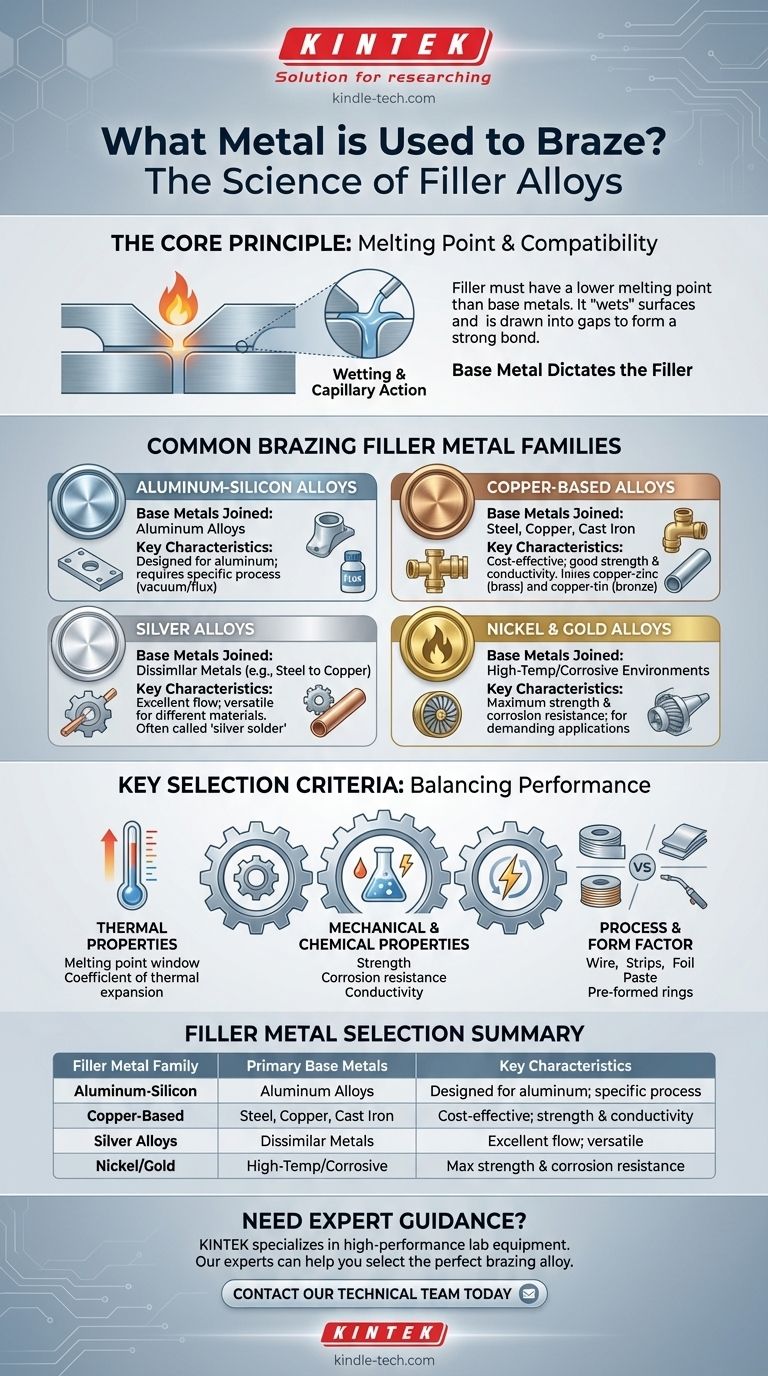
Brazing relies on a specialized filler metal, not the metals being joined. The most common families of these filler metals are alloys based on aluminum-silicon, copper (like brass and bronze), silver, and nickel. The specific alloy is chosen based on the materials you are joining and the performance requirements of the final part.
Choosing the right brazing metal isn't about finding a single "best" option. It's about selecting a filler alloy that is chemically compatible with the base metals and has a melting point low enough to join them without melting the parts themselves.

The Core Principle: Melting Point and Compatibility
The fundamental rule of brazing is that the filler metal must have a lower melting point than the base metals being joined. The process involves heating the assembly to a temperature that melts the filler, but not the components.
Understanding "Wetting"
Effective brazing requires the molten filler metal to "wet" the surfaces of the base metals. This means it must flow freely and be drawn into the tight gap between the parts through capillary action, creating a strong, continuous bond upon cooling.
The Base Metal Dictates the Filler
The materials you intend to join—such as steel, copper, or aluminum—are the single most important factor in selecting a filler. An aluminum filler won't properly bond steel, and a copper filler would melt an aluminum part.
Common Brazing Filler Metal Families
While countless specific alloys exist, they generally fall into a few key categories, each suited for different applications and base metals.
Aluminum-Silicon Alloys
These are used almost exclusively for brazing aluminum and its alloys. The specific composition can vary depending on the brazing process. For example, some alloys contain magnesium to help break through aluminum oxide in a vacuum furnace, while others are designed to be used with a chemical flux.
Copper-Based Alloys
This is a very broad and widely used category. Alloys like copper-zinc (brass) and copper-tin (bronze) are workhorses in the industry. They are excellent for joining carbon steel, stainless steel, cast iron, and copper itself. They offer good strength, corrosion resistance, and high electrical and thermal conductivity.
Silver Alloys
Often called "silver solder" (though this is technically incorrect), silver-based brazing alloys are prized for their versatility. They have excellent flow characteristics and can join a wide variety of dissimilar metals, such as copper to steel.
Nickel and Gold Alloys
These alloys are reserved for high-performance applications. Nickel alloys provide exceptional strength and corrosion resistance at elevated temperatures, making them suitable for aerospace and industrial turbine components. Gold-based alloys offer superior corrosion resistance and are used in specialized electronic or medical devices.
Understanding the Trade-offs: Key Selection Criteria
Choosing a filler metal involves balancing several technical requirements against the final goal for the product.
Thermal Properties
The filler's melting point must be in a precise window—low enough to not damage the base metal, but high enough to ensure the final joint has sufficient strength for its service temperature. Additionally, the filler's coefficient of thermal expansion should be close to that of the base metal to prevent internal stress and cracking as the part cools.
Mechanical and Chemical Properties
The final brazed joint must meet the product's needs. Will it be under high stress? Does it need to resist a specific chemical? Does it need to conduct electricity? The filler metal is a permanent part of the assembly and must possess the required strength, corrosion resistance, or conductivity.
Process and Form Factor
Filler metals are available in various forms, including wire, strips, foil, paste, and pre-formed rings. The choice of form factor depends on the joint design and the manufacturing process (e.g., automated furnace brazing vs. manual torch brazing).
Making the Right Choice for Your Application
Your final selection is a direct function of your materials and your end goal.
- If your primary focus is joining aluminum components: Use an aluminum-silicon filler alloy, ensuring its composition is suited for your specific brazing process (e.g., vacuum vs. flux).
- If your primary focus is joining steel, copper, or cast iron: Copper-based alloys like brass are a versatile and cost-effective choice offering good strength and conductivity.
- If your primary focus is joining dissimilar metals (e.g., steel to copper): Silver-based brazing alloys are often the ideal solution due to their excellent wetting characteristics across different materials.
- If your primary focus is high-temperature performance or extreme corrosion resistance: Investigate nickel or precious metal-based alloys designed specifically for these demanding environments.
Ultimately, selecting the correct filler metal is a critical engineering decision that directly dictates the strength, longevity, and performance of the final assembly.
Summary Table:
| Filler Metal Family | Primary Base Metals Joined | Key Characteristics |
|---|---|---|
| Aluminum-Silicon | Aluminum Alloys | Designed for aluminum; requires specific process (vacuum/flux) |
| Copper-Based (Brass, Bronze) | Steel, Copper, Cast Iron | Cost-effective; good strength & conductivity |
| Silver Alloys | Dissimilar Metals (e.g., Steel to Copper) | Excellent flow; versatile for different materials |
| Nickel/Gold Alloys | High-Temp/Corrosive Environments | Maximum strength & corrosion resistance; for demanding applications |
Need expert guidance selecting the perfect brazing alloy for your specific materials and performance requirements?
KINTEK specializes in high-performance lab equipment and consumables, serving the precise needs of laboratories and R&D facilities. Our experts can help you navigate the complexities of filler metal selection to ensure your brazed joints achieve optimal strength, durability, and performance.
Contact our technical team today to discuss your project and discover the right brazing solution for you.
Visual Guide
Related Products
- Copper Foam
- Thermally Evaporated Tungsten Wire for High Temperature Applications
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Optical Window Glass Substrate Wafer Single Double Sided Coated K9 Quartz Sheet
- Custom PTFE Teflon Parts Manufacturer for Hollow Cleaning Basket and Rack Carrier
People Also Ask
- How can different materials have different heat capacity? Unlocking the Microscopic Secrets of Energy Storage
- What role does convection play in heat transfer? Understanding Heat Movement in Fluids
- What are the characteristics of copper foam? Unlock High-Performance Thermal and Electrical Solutions
- Can I solder copper to copper without flux? The Critical Role of Flux for a Strong Bond
- What are the available sizes and thicknesses for copper foam? Optimize Your Thermal and Filtration Performance
















