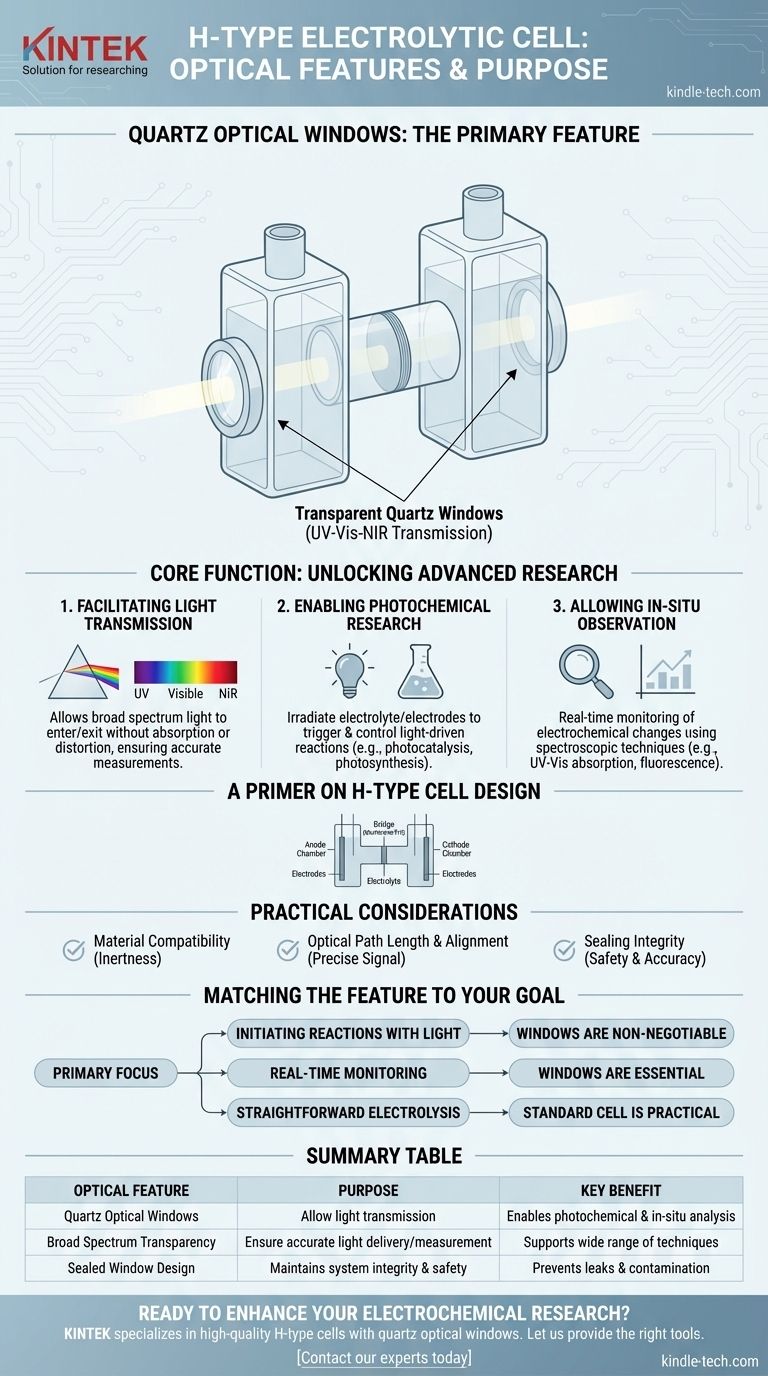
The primary optical feature of an H-type electrolytic cell is the inclusion of quartz optical windows. These windows are specifically designed to be transparent, allowing light to easily enter and exit the cell's compartments. This transforms the device into a tool for advanced photochemical and spectroelectrochemical research.
The integration of quartz windows fundamentally changes the cell's purpose. It moves beyond simple electrolysis, enabling researchers to use light as a tool to either trigger a reaction or to observe a reaction as it unfolds in real time.

The Core Function of Optical Windows
The addition of transparent windows to an H-type cell is a deliberate design choice that unlocks new experimental possibilities by bridging the fields of optics and electrochemistry.
Facilitating Light Transmission
The windows are made of quartz because it is highly transparent across a broad spectrum of light, including ultraviolet (UV), visible, and near-infrared (NIR) wavelengths.
This material choice is critical, as it ensures that the light used for an experiment is not absorbed or distorted by the window itself, allowing for accurate measurements.
Enabling Photochemical Research
These windows allow researchers to irradiate the electrolyte or electrode surfaces with a light source.
This capability is essential for studying photocatalysis, photosynthesis, or any reaction that is initiated or influenced by light. You can precisely control when and how a reaction starts by controlling the light.
Allowing In-Situ Observation
"In-situ" means "in the original place." The windows allow for real-time monitoring of the electrochemical reaction as it happens, without disturbing the system.
By passing a light beam through the cell and into a detector, scientists can use techniques like UV-Vis absorption or fluorescence spectroscopy to measure changes in the chemical species inside the cell during the experiment.
A Primer on the H-Type Cell Design
To understand why optical windows are so valuable, it helps to understand the fundamental structure of the H-type cell itself.
The Two-Chamber System
The "H" shape comes from its design, which features two separate compartments or half-cells, one for the anode and one for the cathode. These are typically connected by a bridge containing a membrane or frit.
This separation is crucial because it prevents the products generated at one electrode from migrating to the other and causing unwanted side reactions.
Standard Electrochemical Components
Like any electrolytic cell, the H-type design includes two electrodes (the anode and cathode) and an electrolyte. The electrolyte is an ion-conducting solution that fills the chambers and allows charge to flow between the electrodes, completing the electrical circuit.
Understanding the Practical Considerations
While powerful, using an H-type cell with optical windows requires attention to detail to ensure accurate and repeatable results.
Material Compatibility
The quartz windows and the cell body must be chemically inert to the electrolyte and solvents used in the experiment. Any chemical reaction with the cell itself would compromise the results.
Optical Path Length and Alignment
The distance the light travels through the sample (the optical path length) is a critical parameter for many spectroscopic measurements. The design of the cell dictates this length, and the external light source and detector must be precisely aligned with the windows for an accurate signal.
Sealing Integrity
The seal between the quartz window and the cell body must be perfect. Any leak can not only ruin a sensitive experiment but also pose a potential safety hazard depending on the chemicals being used.
Matching the Feature to Your Research Goal
The decision to use an H-type cell with optical windows should be driven by your specific experimental needs.
- If your primary focus is initiating reactions with light: The windows are non-negotiable, serving as the delivery port for the photons needed to drive your photochemical process.
- If your primary focus is real-time monitoring of a reaction: The windows are essential for applying in-situ spectroscopic techniques to track how chemical concentrations change over time.
- If your primary focus is straightforward electrolysis without light interaction: A standard H-type cell without optical windows is a more practical and cost-effective choice.
Ultimately, these optical windows serve to open up a closed system, allowing you to see and control electrochemical reactions in ways that would otherwise be impossible.
Summary Table:
| Optical Feature | Purpose | Key Benefit |
|---|---|---|
| Quartz Optical Windows | Allow light transmission into/out of the cell | Enables photochemical reactions and in-situ spectroscopic analysis |
| Broad Spectrum Transparency (UV-Vis-NIR) | Ensure accurate light delivery and measurement | Supports a wide range of light-based techniques without signal distortion |
| Sealed Window Design | Maintains system integrity and safety | Prevents leaks and contamination during sensitive experiments |
Ready to enhance your electrochemical research with precision optical capabilities?
KINTEK specializes in high-quality lab equipment, including specialized H-type electrolytic cells with quartz optical windows. Our products are designed to meet the rigorous demands of photochemical and spectroelectrochemical studies, ensuring accurate light transmission, material compatibility, and reliable sealing for your most critical experiments.
Let us provide the right tools for your laboratory's success.
Contact our experts today to discuss your specific application needs and discover how our solutions can drive your research forward.
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
People Also Ask
- How should failures or malfunctions of an H-type electrolytic cell be handled? A Guide to Safe and Effective Troubleshooting
- What are the proper storage conditions for an H-type electrolytic cell? Ensure Long-Term Reliability and Accurate Results
- What are the key safety operation guidelines for using the H-type electrolytic cell? Best Practices for Your Lab
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What experimental conditions need to be controlled when using an H-type electrolytic cell? Ensure Reliable and Repeatable Results



















