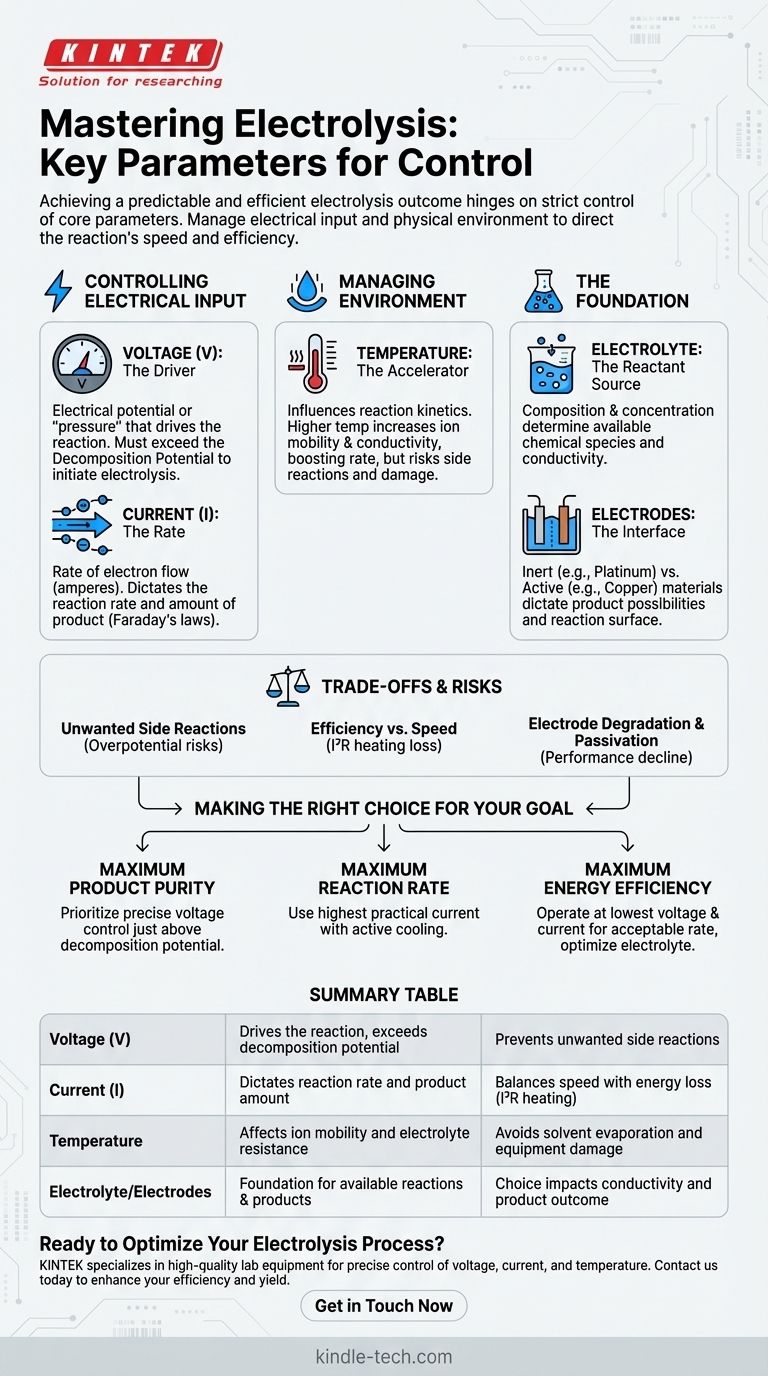
In any electrolysis setup, achieving a predictable and efficient outcome hinges on the strict control of several core parameters. To ensure the reaction proceeds as intended, you must precisely manage the electrical input—specifically voltage and current—as well as the physical environment, primarily the temperature of the electrolyte. These three variables are the primary levers for controlling the reaction's speed and efficiency.
The core challenge of electrolysis is not merely initiating a reaction, but directing it. While controlling voltage, current, and temperature is the immediate task, true mastery comes from understanding how these parameters, along with your choice of electrolyte and electrodes, dictate the final products and overall efficiency.

The Pillars of Electrolytic Control
Successful electrolysis is a balancing act. Each parameter influences the others and has a direct impact on the final result. Understanding their individual roles is the first step toward process mastery.
Controlling Electrical Input: Voltage and Current
The electrical supply is the engine of your reaction. Voltage (V) is the electrical potential or "pressure" that drives the reaction. It must be sufficient to overcome the decomposition potential of the electrolyte, which is the minimum voltage required for electrolysis to begin.
Current (I), measured in amperes, is the rate of electron flow. According to Faraday's laws of electrolysis, the amount of chemical substance produced is directly proportional to the amount of current passed through the system over time. In short, current dictates the reaction rate.
Managing the Reaction Environment: Temperature
Temperature influences the reaction kinetics. Increasing the temperature generally lowers the solution's viscosity and increases the mobility of ions, which in turn decreases the electrical resistance of the electrolyte.
This allows for a higher reaction rate at a given voltage. However, excessive heat can cause unwanted side reactions, lead to the evaporation of the solvent, or even damage the equipment.
The Foundation: Electrolyte and Electrodes
While not dynamic process variables in the same way as temperature or current, the initial choice of electrolyte and electrodes is the most fundamental act of control you have.
The electrolyte's composition and concentration determine which chemical species are available to react. Higher concentrations typically increase conductivity but can also alter which reactions are favored at the electrodes.
The electrode material determines whether the electrode simply provides a surface for the reaction (inert electrodes like platinum or graphite) or participates in it directly (active electrodes like copper or zinc), fundamentally changing the possible products.
Understanding the Trade-offs and Risks
Controlling electrolysis is not just about setting dials; it's about navigating compromises and avoiding common pitfalls that can ruin your results.
The Risk of Unwanted Side Reactions
Applying a voltage significantly higher than the decomposition potential is a common mistake. This "overpotential" can provide enough energy to trigger unintended reactions, such as the electrolysis of water itself, which reduces the current efficiency for your target product.
Efficiency vs. Speed
There is a constant trade-off between speed and energy efficiency. Pushing for a high reaction rate by increasing the current also increases resistive heat losses (I²R heating). This wastes energy and necessitates more robust temperature control, adding complexity and cost.
Electrode Degradation and Passivation
Even electrodes considered "inert" can degrade under extreme conditions of voltage or current density. More critically, the surface of an electrode can become coated with an insulating layer of product—a phenomenon called passivation—which can slow or completely halt the electrolysis process.
Making the Right Choice for Your Goal
The optimal control strategy depends entirely on what you want to achieve. Use your primary objective to guide how you manage the key parameters.
- If your primary focus is maximum product purity: Prioritize precise voltage control to stay just above the decomposition potential for your target reaction, preventing side reactions.
- If your primary focus is maximum reaction rate: Use the highest practical current your system can handle while implementing an active cooling system to manage the resulting temperature increase.
- If your primary focus is maximum energy efficiency: Operate at the lowest possible voltage and current that still yields an acceptable rate, and optimize electrolyte concentration to minimize resistance.
Mastering these parameters transforms electrolysis from a simple demonstration into a precise and predictable chemical manufacturing tool.
Summary Table:
| Parameter | Primary Role | Key Consideration |
|---|---|---|
| Voltage (V) | Drives the reaction, must exceed decomposition potential | Prevents unwanted side reactions |
| Current (I) | Dictates reaction rate and product amount | Balances speed with energy loss (I²R heating) |
| Temperature | Affects ion mobility and electrolyte resistance | Avoids solvent evaporation and equipment damage |
| Electrolyte/Electrodes | Foundation for available reactions and products | Choice impacts conductivity and product outcome |
Ready to Optimize Your Electrolysis Process?
Achieving precise control over voltage, current, and temperature is critical for efficient and predictable results. Whether your goal is maximum product purity, high reaction speed, or optimal energy efficiency, the right lab equipment is essential.
KINTEK specializes in high-quality lab equipment and consumables, serving all your laboratory needs. From precise power supplies to temperature control systems, we provide the reliable tools you need to master your electrolysis parameters.
Contact us today to discuss how our solutions can enhance your process efficiency and yield. Let's build a more controlled and productive lab together.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the necessary steps to prepare an all-quartz electrolytic cell before an experiment? Ensure Accuracy and Safety
- What is the proper procedure for post-experiment cleanup and storage of an all-quartz electrolytic cell? Ensure Longevity and Reproducibility
- What materials are used to construct the all-quartz electrolytic cell? A Guide to Purity and Performance
- What precautions should be taken when handling and using an all-quartz electrolytic cell? Ensure Safe, Accurate, and Durable Performance
- What are the available volumes and dimensions for the all-quartz electrolytic cell? Find the Perfect Fit for Your Lab



















