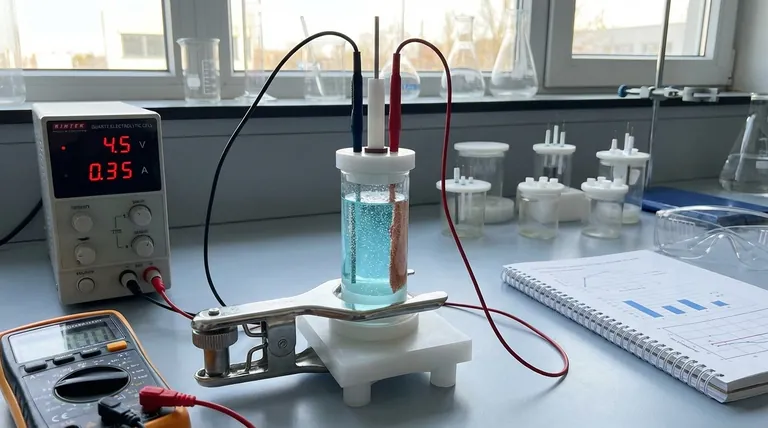
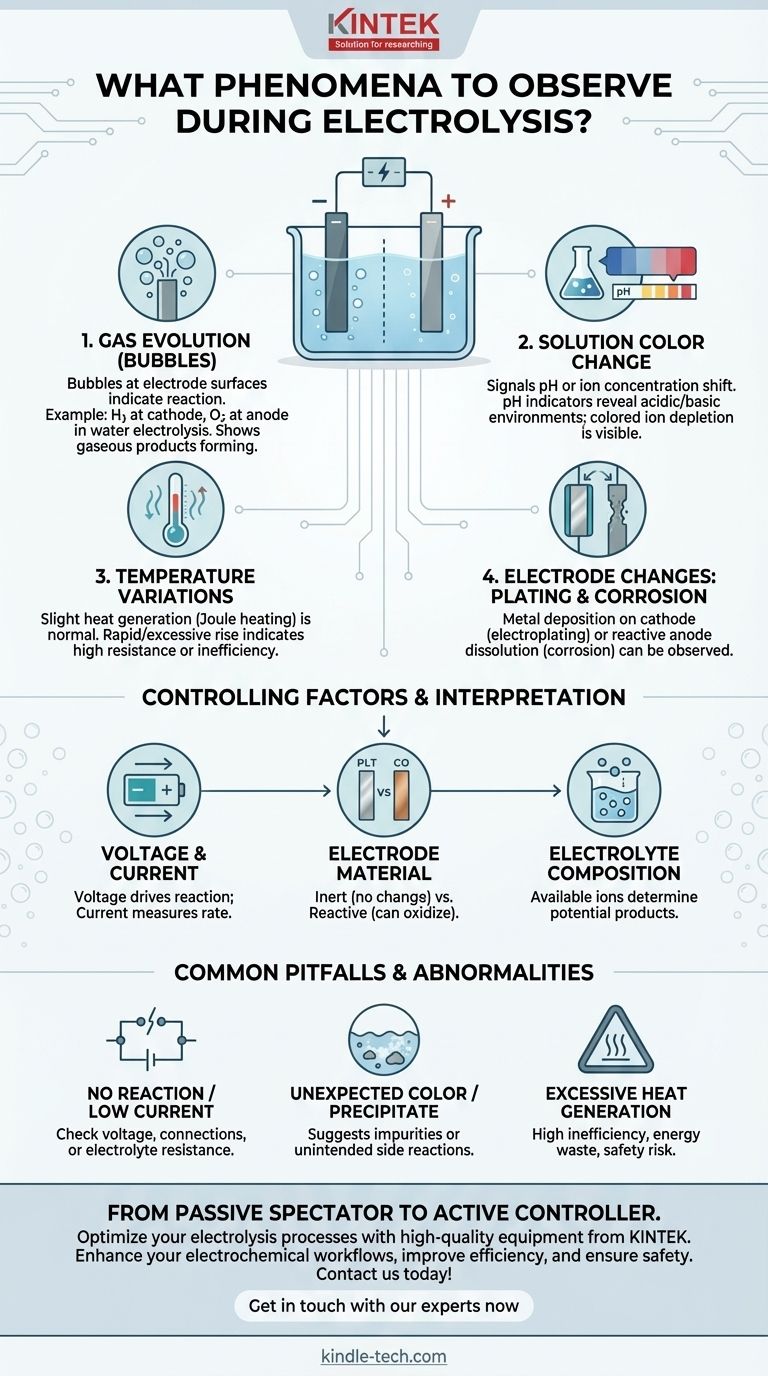
When conducting electrolysis, the primary phenomena to observe are the generation of gas bubbles at the electrode surfaces, changes in the solution's color, and any variation in temperature. These visual and physical cues are direct indicators of the underlying electrochemical reaction, providing immediate feedback on whether the process is proceeding as expected.
Observing electrolysis is more than just watching bubbles form. Each phenomenon is a piece of data that reveals the reaction's identity, efficiency, and safety, allowing you to interpret what is happening at the molecular level.
Interpreting Key Observations
Each observable event during electrolysis provides a clue about the chemical transformations taking place. Understanding what these clues mean is essential for controlling the outcome.
Gas Evolution (Bubbles) at the Electrodes
Bubbles are the most common and immediate sign that a reaction is occurring. These are gaseous products being formed directly on the electrode surfaces.
For example, in the electrolysis of water, you will see hydrogen gas bubbling at the negative electrode (cathode) and oxygen gas at the positive electrode (anode). The specific gas produced depends entirely on the electrolyte's composition.
Changes in Solution Color
A color change in the electrolyte can signal several different events. It often indicates a change in pH or a change in the concentration of specific ions.
If a pH indicator (like litmus or universal indicator) is present, color changes near the electrodes reveal the formation of acidic or basic environments. Alternatively, if the electrolyte contains colored ions (like blue copper(II) ions), their depletion or formation can be tracked visually.
Temperature Variations
Electrolysis involves passing an electrical current through a solution, which inherently generates some heat due to electrical resistance (Joule heating).
A slight, gradual increase in temperature is normal. However, a rapid or excessive temperature rise can indicate high internal resistance, an inefficient process, or an unintended and highly exothermic side reaction.
Electrode Changes: Plating or Corrosion
The electrodes themselves can change. In processes like electroplating, you will observe a layer of metal depositing and growing on the cathode.
Conversely, if a reactive anode (like copper or zinc) is used, you may see it visibly corrode or dissolve into the solution as it is oxidized. Inert electrodes, like platinum or carbon, should show no change.
Understanding the Controlling Factors
The phenomena you observe are directly controlled by the parameters you set. The type of electrode and electrolyte determine what can happen, while voltage and current determine if it happens and how fast.
Voltage and Current
Voltage is the driving force of the reaction; a minimum voltage (the decomposition potential) must be applied for electrolysis to begin.
Current is a measure of the rate of the reaction. It is directly proportional to the rate at which electrons are being transferred and, therefore, the rate at which products are being formed.
Electrode Material (Inert vs. Reactive)
Inert electrodes (e.g., platinum, graphite) serve only as a surface for the reaction to occur without participating chemically.
Reactive electrodes (e.g., copper, zinc, nickel) can be oxidized at the anode, entering the solution as ions. This is a fundamental principle in electrorefining and electroplating.
Electrolyte Composition
The ions available in the electrolyte determine the potential products. In a solution with multiple types of ions, the one that is easiest to reduce will react at the cathode, and the one that is easiest to oxidize will react at the anode.
Common Pitfalls and Abnormal Situations
Observing the process allows you to identify when things are not working correctly. These "abnormal situations" are critical diagnostic tools.
No Reaction or Very Low Current
This typically indicates a problem with the setup. The cause could be insufficient voltage, a poor electrical connection, or an electrolyte with very high resistance.
Unexpected Color or Precipitate
If you observe a color not predicted by the main reaction or see a solid (precipitate) forming in the solution, it strongly suggests the presence of impurities. This indicates that an unintended side reaction is occurring.
Excessive Heat Generation
As mentioned, significant heat points to high inefficiency. It means a large portion of the electrical energy is being wasted as heat instead of being used to drive the desired chemical change. This can also pose a safety risk.
Making the Right Choice for Your Goal
Your interpretation of these observations depends on the goal of your experiment.
- If your primary focus is demonstrating a basic principle (e.g., water electrolysis): Look for the classic signs of gas bubbling at both electrodes and use a pH indicator to see the formation of base at the cathode and acid at the anode.
- If your primary focus is electroplating: The most important observation is the uniform deposition of metal onto the cathode, with current and time being the key control parameters.
- If your primary focus is quantitative analysis (e.g., verifying Faraday's Laws): You must ensure a constant, stable current, as this directly relates the amount of product formed to the total charge passed through the cell.
By carefully observing these phenomena, you transition from a passive spectator to an active controller of the electrochemical process.
Summary Table:
| Phenomenon | What It Indicates | Key Insight |
|---|---|---|
| Gas Bubbles | Reaction occurring; product formation (e.g., H₂ at cathode, O₂ at anode in water) | Identifies the gaseous products of the reaction |
| Color Change | pH shift or ion concentration change (e.g., with indicators or colored ions like Cu²⁺) | Reveals chemical environment and reaction progress |
| Temperature Rise | Joule heating; excessive heat may indicate inefficiency or side reactions | Monitors process safety and energy efficiency |
| Electrode Plating/Corrosion | Metal deposition (cathode) or dissolution (anode) in processes like electroplating | Critical for applications requiring surface modification |
Optimize Your Electrolysis Processes with KINTEK
Are you looking to achieve precise control over your electrolysis experiments or industrial applications? Understanding these observable phenomena is just the first step. KINTEK specializes in high-quality lab equipment and consumables, including reliable electrodes, stable power supplies, and durable electrolysis cells designed for consistent performance and accurate results.
Whether you're conducting research, developing new materials, or scaling up production, our products help you monitor and control key parameters effectively. Contact us today to discuss how our solutions can enhance your electrochemical workflows, improve efficiency, and ensure safety.
Get in touch with our experts now to find the perfect equipment for your laboratory needs!
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What is the proper procedure for post-experiment cleanup and storage of an all-quartz electrolytic cell? Ensure Longevity and Reproducibility
- Why is a quartz electrolytic cell used for acrylic acid wastewater? Ensure Chemical Stability & Data Integrity
- What are the operational procedures and safety precautions during an experiment using an all-quartz electrolytic cell? Ensure Safety and Accuracy in Your Lab
- What are the available volumes and dimensions for the all-quartz electrolytic cell? Find the Perfect Fit for Your Lab
- How should an all-quartz electrolytic cell and its components be maintained for long-term use? A Guide to Maximizing Equipment Lifespan



















