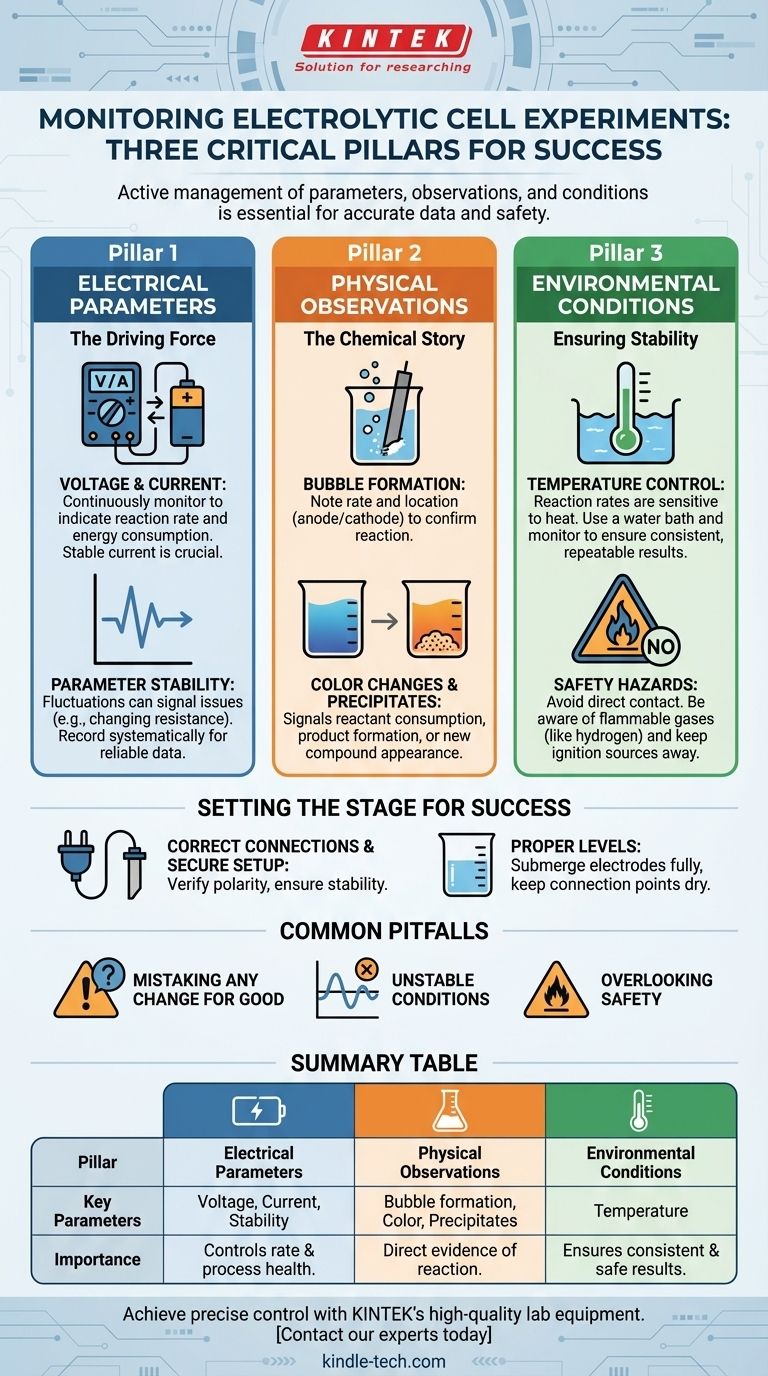
During an experiment, you must monitor three critical aspects of the electrolytic cell: the electrical parameters driving the reaction (voltage, current), the physical changes that reveal the chemical process (bubble formation, color changes), and the environmental conditions that ensure stability (temperature). These observations are essential for collecting accurate data, confirming the reaction is proceeding as intended, and addressing any issues promptly.
The core purpose of monitoring an electrolytic cell is not just to record data, but to actively manage the experiment. It allows you to ensure the chemical reaction you want is the one that's actually happening, safely and efficiently.

The Pillars of Effective Monitoring
To run a successful electrolysis experiment, your monitoring strategy should be built on three distinct but interconnected pillars. Each provides a different window into the process.
Electrical Parameters: The Driving Force
Your power supply's settings are the direct inputs controlling the reaction.
Voltage and Current These are not set-and-forget values. They must be monitored continuously as they indicate the rate and energy consumption of your reaction. A stable current is often crucial for quantitative experiments.
Parameter Stability Fluctuations in voltage or current can signal issues like changing electrolyte resistance or electrode surface degradation. Recording these values systematically is key to producing reliable data.
Physical Observations: The Chemical Story
What you see is direct evidence of the chemical transformation taking place inside the cell.
Bubble Formation The generation of gas bubbles on the electrode surfaces is a primary indicator of electrolysis. Note the location (anode vs. cathode) and rate of bubble formation to confirm your expected reaction.
Color Changes A change in the electrolyte's color often signifies the consumption of a reactant or the formation of a new product. This is a powerful qualitative confirmation that your synthesis is working.
Precipitate Formation The appearance of a solid material (a precipitate) is another clear signal of a chemical reaction occurring, which should be noted.
Environmental Conditions: Ensuring Stability
The environment in which the reaction occurs can significantly impact the outcome.
Temperature Control Electrolytic reactions can generate heat, and reaction rates are highly sensitive to temperature. Using a constant temperature water bath and monitoring the electrolyte's temperature is critical for consistent and repeatable results.
Setting the Stage for Success
Effective monitoring is impossible without a correct and safe initial setup. This foundation prevents equipment damage and ensures the data you collect is meaningful.
Correct Electrical Connections
Always double-check that the positive and negative terminals of your power supply are connected to the correct electrodes (anode and cathode). Reversing the polarity can damage the cell or ruin the experiment.
Secure Mechanical Setup
The cell must be stable. Secure it on a stand to ensure it remains vertical and does not wobble. If using corrosive electrolytes, place a leak-proof pad underneath as a safety precaution.
Proper Electrode and Electrolyte Levels
Ensure electrodes are installed with appropriate spacing and are fully submerged in the electrolyte. However, the connection points of the electrode rods should remain above the liquid to prevent corrosion and short-circuiting.
Common Pitfalls and How to Avoid Them
Monitoring is about more than just watching; it's about interpreting what you see and avoiding common mistakes.
Mistaking Any Change for a Good Change
Not all observations are positive. Unwanted side reactions can also produce gas or color changes. Compare your observations against the expected outcome of your specific chemical reaction.
The Risk of Unstable Conditions
Failing to control temperature or allowing the current to drift will introduce variables that make your results unreliable. The goal is to isolate the variable you are studying by keeping all other conditions constant.
Overlooking Safety Hazards
Monitoring extends to the lab environment. Never touch electrodes or the electrolyte directly. Be aware that some reactions (like the electrolysis of water) produce flammable gases (hydrogen), so keep all open flames or spark sources away from the apparatus.
Tailoring Your Monitoring to Your Goal
The focus of your monitoring should align with the primary objective of your experiment.
- If your primary focus is quantitative analysis: Prioritize the precise recording and stabilization of the electrical current and reaction time.
- If your primary focus is chemical synthesis: Concentrate on the qualitative physical observations—color changes and product formation—that confirm the target reaction is happening.
- If your primary focus is process efficiency or safety: Pay closest attention to temperature fluctuations and the rate of gas evolution to prevent runaway reactions.
Diligent monitoring transforms an experiment from a passive procedure into an actively controlled and understood scientific investigation.
Summary Table:
| Monitoring Pillar | Key Parameters to Track | Why It's Important |
|---|---|---|
| Electrical Parameters | Voltage, Current, Stability | Controls reaction rate and indicates process health. |
| Physical Observations | Bubble formation, Color changes, Precipitates | Provides direct evidence of the chemical reaction. |
| Environmental Conditions | Temperature | Ensures consistent, repeatable, and safe results. |
Achieve precise control and reliable data in your electrolysis experiments. KINTEK specializes in high-quality lab equipment and consumables for all your laboratory needs. From stable power supplies to durable electrodes and safe cell setups, our products are designed to support your scientific investigations. Contact our experts today to find the right equipment for your specific application and enhance your lab's efficiency and safety.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Flat Corrosion Electrolytic Electrochemical Cell
People Also Ask
- What is the proper procedure for post-experiment cleanup and storage of an all-quartz electrolytic cell? Ensure Longevity and Reproducibility
- Why is a quartz electrolytic cell used for acrylic acid wastewater? Ensure Chemical Stability & Data Integrity
- What materials are used to construct the all-quartz electrolytic cell? A Guide to Purity and Performance
- What are the necessary steps to prepare an all-quartz electrolytic cell before an experiment? Ensure Accuracy and Safety
- What are the available volumes and dimensions for the all-quartz electrolytic cell? Find the Perfect Fit for Your Lab



















