To prevent material failure, you must avoid exposing acrylic electrolytic cells to strong organic solvents. The most common examples to forbid from contact are ketones like acetone and halogenated hydrocarbons such as chloroform, as these chemicals will rapidly cause the acrylic to crack, swell, or craze, leading to irreversible damage.
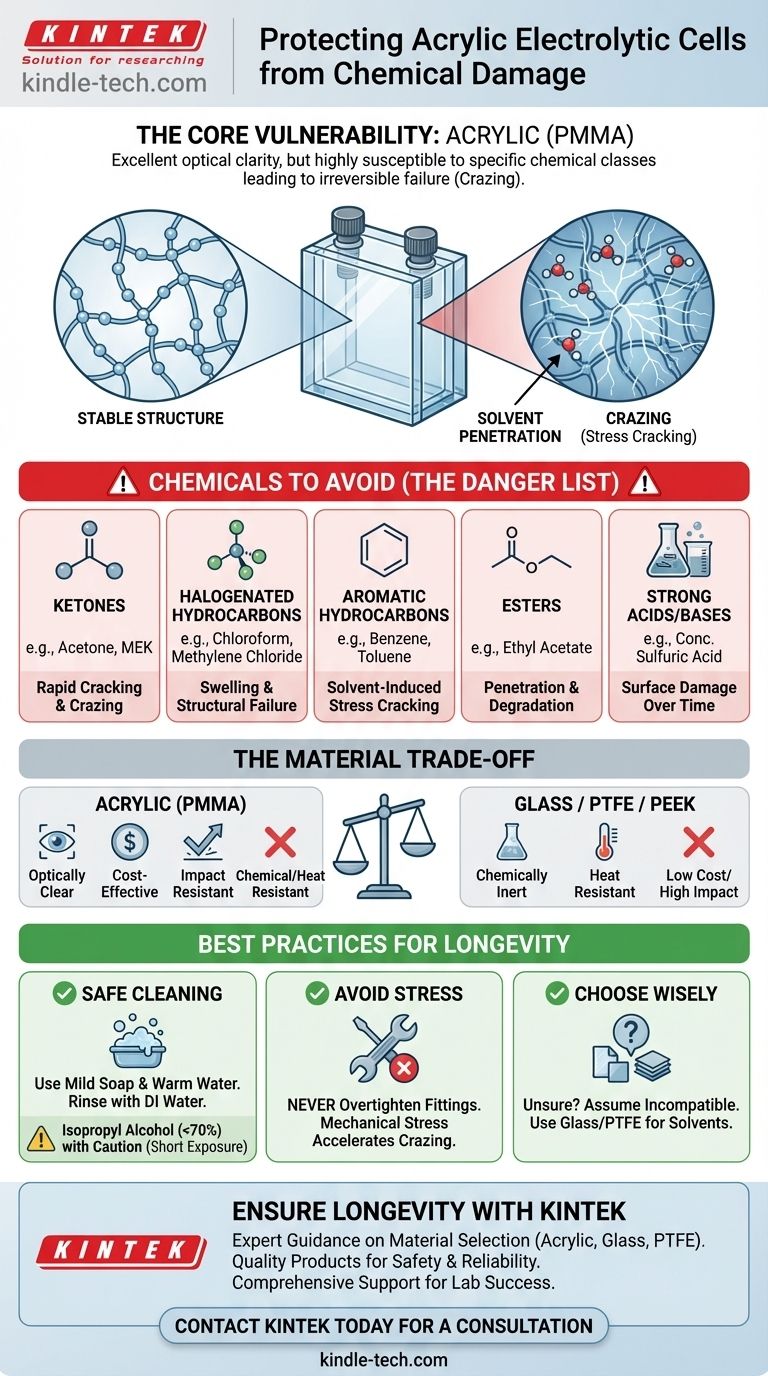
The core issue is that acrylic (PMMA), while optically clear and affordable, has a polymer structure that is highly vulnerable to specific chemical classes. Understanding which substances to avoid is not just about a list of chemicals, but about recognizing the fundamental incompatibility between the material and certain solvents.
Understanding Acrylic's Vulnerability
Acrylic, scientifically known as poly(methyl methacrylate) or PMMA, is a transparent thermoplastic. Its widespread use in laboratory equipment like electrolytic cells is due to its excellent optical clarity, ease of fabrication, and lower cost compared to glass. However, this material has distinct chemical limitations.
The Mechanism of Chemical Attack
The primary mode of failure for acrylic under chemical exposure is solvent-induced stress cracking, often called crazing. This isn't just a surface issue; it's a structural failure.
When an aggressive solvent touches the acrylic, its molecules penetrate the polymer chains. This causes the material to swell and plasticize, weakening the bonds between the polymer chains. If the material is also under any mechanical stress (even residual stress from manufacturing), microscopic cracks will form and propagate, appearing as a network of fine white lines.
Key Chemical Classes to Avoid
While acetone and chloroform are the most cited examples, the list of damaging substances is broader. You should treat the following chemical families with extreme caution:
- Ketones: Acetone, methyl ethyl ketone (MEK)
- Halogenated Hydrocarbons: Chloroform, methylene chloride, carbon tetrachloride
- Aromatic Hydrocarbons: Benzene, toluene, xylene
- Esters: Ethyl acetate, methyl methacrylate (the monomer of acrylic itself)
- Strong Acids and Bases: While having better resistance than some plastics, concentrated acids and alkalis can still cause damage over time.
The Trade-offs of Using Acrylic Cells
Understanding a material's weaknesses is key to using it effectively. Acrylic is chosen for specific reasons, and its limitations are the trade-off for those benefits.
Benefit: Optical Clarity and Cost
Acrylic's primary advantage is its near-perfect optical transparency (up to 92% light transmission), which is often superior to glass. This allows for clear visual observation of electrochemical processes, which is critical in research and educational settings. It is also significantly less expensive and more impact-resistant than glass or quartz cells.
Limitation: Chemical and Thermal Sensitivity
The key trade-off is chemical resistance. Unlike borosilicate glass, which is inert to most chemicals, acrylic is highly selective. It is also sensitive to heat, with a low continuous service temperature, meaning high-temperature experiments are not feasible.
Best Practices for Handling and Cleaning
Proper care is the most effective way to ensure the long life of your acrylic equipment.
Safe Cleaning Agents
For routine cleaning, use a soft cloth with mild soap or detergent and lukewarm water. Rinse thoroughly with deionized water and allow it to air dry. For disinfection or removing stubborn residues, isopropyl alcohol (IPA) can often be used, but with caution. Use a lower concentration (<70%) and limit exposure time, as prolonged contact can still cause crazing.
Avoiding Mechanical Stress
Never overtighten fittings or clamps on an acrylic cell. Mechanical stress dramatically lowers the threshold for chemical attack. Even a solvent that might be considered safe can cause crazing if the material is under tension.
Making the Right Choice for Your Goal
Protecting your equipment requires matching the material to the task.
- If your primary focus is safe cleaning and maintenance: Stick to mild soap, deionized water, and approved acrylic-safe cleaners.
- If your experiment involves organic solvents: An acrylic cell is likely the wrong material. You must switch to a cell made of borosilicate glass, PTFE, or PEEK.
- If you are unsure about a chemical's compatibility: Assume it is incompatible. Test on a small, non-critical area first or consult a detailed chemical compatibility chart from a reputable source.
Ultimately, treating your acrylic equipment with an awareness of its material properties is the best way to protect your investment and ensure reliable results.

Summary Table:
| Chemical Class | Examples to Avoid | Primary Risk to Acrylic (PMMA) |
|---|---|---|
| Ketones | Acetone, Methyl Ethyl Ketone (MEK) | Rapid cracking and crazing |
| Halogenated Hydrocarbons | Chloroform, Methylene Chloride | Swelling and structural failure |
| Aromatic Hydrocarbons | Benzene, Toluene, Xylene | Solvent-induced stress cracking |
| Esters | Ethyl Acetate | Penetration and polymer degradation |
| Strong Acids/Bases | Concentrated Sulfuric Acid, Sodium Hydroxide | Potential surface damage over time |
Ensure the Longevity of Your Lab Equipment with KINTEK
Protecting your acrylic electrolytic cells from chemical damage is crucial for reliable experiments and cost-effective lab management. At KINTEK, we specialize in providing the right laboratory equipment and expert guidance to match your specific application needs.
Why choose KINTEK?
- Expert Guidance: Our specialists can help you select the perfect electrolytic cell material—whether acrylic, borosilicate glass, or advanced polymers like PTFE—for your specific chemical processes.
- Quality Products: We supply durable, high-performance lab equipment designed for safety and longevity.
- Comprehensive Support: From chemical compatibility advice to maintenance best practices, we are your partner in achieving accurate and consistent results.
Don't risk material failure. If your experiments involve aggressive solvents or you need advice on equipment care, our team is here to help.
Contact KINTEK today for a consultation and ensure your lab operates with precision and reliability!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results



















