In practice, diffusion bonding occurs at a temperature between 50% and 80% of the absolute melting point (Tm) of the parent material. For example, to bond titanium alloys with a melting point around 1660°C (1933 K), the process is typically performed between 850°C and 1000°C. The exact temperature is not a fixed number but is carefully selected based on the specific material, the required bond strength, and the other process parameters.
The ideal temperature for diffusion bonding is a carefully controlled variable. It must be high enough to energize atoms to migrate across the joint interface but low enough to prevent melting, unwanted deformation, or degradation of the material's properties.
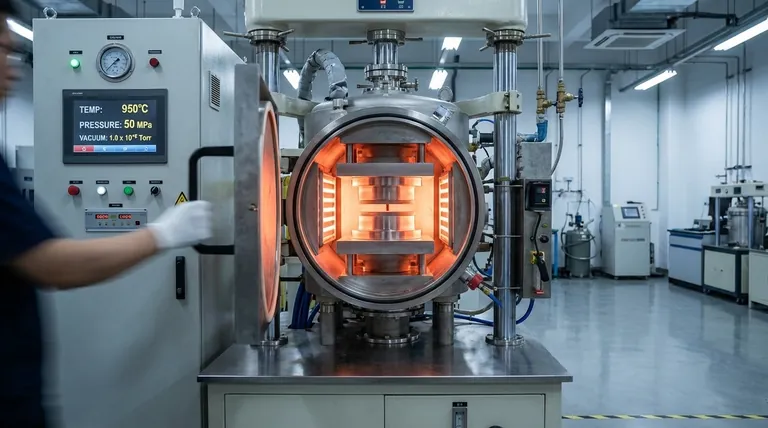
The Role of Temperature in Atomic Diffusion
Diffusion bonding is a solid-state process, meaning no melting occurs. The goal is to encourage atoms from two separate components to move across the boundary and form a single, monolithic piece. Temperature is the primary catalyst for this process.
Activating Atomic Movement
Heat provides the thermal energy that atoms need to overcome their energy barriers and jump from their lattice sites. As temperature increases, atomic vibration and mobility increase exponentially, dramatically accelerating the rate of diffusion across the interface.
Enabling Intimate Contact
Even highly polished surfaces are rough on a microscopic level, covered in peaks (asperities) and valleys. Applying heat softens the material, allowing the applied pressure to more easily deform these peaks. This "creep" mechanism is essential for closing the gaps and achieving the intimate, atom-to-atom contact required for bonding.
Why Not Just Melt It?
Staying below the melting point is the defining feature of diffusion bonding. This preserves the original, fine-grained microstructure of the material, avoiding the defects, residual stresses, and brittleness that can occur during the solidification of a weld. This is critical for high-performance and safety-critical applications.
It's Not Just About Temperature: The Other Critical Parameters
Temperature is only one part of an interconnected system. Achieving a successful bond requires precise control over three other key factors.
The Role of Pressure
A constant pressure is applied across the components during the heating cycle. Its primary job is not to forge the parts together but to ensure the two faying surfaces remain in intimate contact. This pressure helps break down any thin, brittle surface oxide layers and encourages the plastic flow needed to eliminate microscopic voids.
The Importance of Time
Diffusion is a slow, time-dependent process. Bonds can form over minutes or, more commonly, several hours. The longer the components are held at temperature and pressure, the more complete the atomic migration will be, leading to the elimination of the original interface and the growth of shared grains across the boundary.
The Non-Negotiable: Surface Preparation
The surfaces to be joined must be exceptionally clean and smooth. Any contaminants, such as oils, dust, or thick oxide layers, will act as a barrier preventing diffusion. A surface finish of Ra < 0.4 μm is often required, typically achieved through fine machining or grinding, followed by a thorough chemical cleaning process.
Controlling the Atmosphere
Because this process occurs at high temperatures, the components are extremely vulnerable to oxidation. To prevent this, diffusion bonding is almost always performed in a high-vacuum chamber or an atmosphere filled with an inert gas, such as Argon.
Understanding the Trade-offs
The parameters of diffusion bonding are not independent; changing one affects the others. Understanding these relationships is key to process optimization.
Temperature vs. Time
This is the most fundamental trade-off. A higher temperature significantly reduces the required bonding time. However, excessive heat can cause undesirable effects like excessive grain growth, which can weaken the material, or unwanted phase transformations in certain alloys. A lower temperature preserves material properties but requires a much longer, and therefore more expensive, cycle time.
Pressure vs. Deformation
While pressure is necessary, too much of it can cause macroscopic plastic deformation, or "creep," changing the final dimensions of the component. The pressure must be carefully chosen to be below the material's yield strength at the bonding temperature. This is especially critical for joining complex, near-net-shape parts where dimensional accuracy is paramount.
Cost vs. Capability
Diffusion bonding produces exceptionally high-quality joints that are often undetectable and possess parent-metal strength. However, the need for specialized vacuum furnaces, long cycle times, and meticulous surface preparation makes it a relatively expensive process compared to conventional welding. The trade-off is performance for cost.
Making the Right Choice for Your Application
Selecting the correct parameters is a balancing act tailored to your specific goal. Use the principles above to guide your decision.
- If your primary focus is preserving a sensitive microstructure: Use the lowest practical temperature (e.g., ~50-60% Tm) and compensate with a significantly longer hold time.
- If your primary focus is joining dissimilar materials: Choose a temperature that is a suitable compromise for both materials, often limited by the one with the lower melting point, and consider using interlayers to promote diffusion or prevent brittle compound formation.
- If your primary focus is maximizing manufacturing throughput: Use the highest temperature the material can tolerate without unacceptable grain growth or distortion (e.g., ~70-80% Tm) to minimize the bonding time.
Ultimately, mastering diffusion bonding comes from viewing temperature, pressure, and time not as isolated settings, but as an interconnected system to be optimized for your specific material and performance requirements.
Summary Table:
| Parameter | Role in Diffusion Bonding | Key Consideration |
|---|---|---|
| Temperature | Primary driver of atomic diffusion and mobility. | Must be high enough for diffusion but below melting point to avoid microstructure damage. |
| Pressure | Ensures intimate contact between surfaces and helps break oxide layers. | Must be sufficient for contact but low enough to prevent unwanted deformation. |
| Time | Allows for complete atomic migration and grain growth across the interface. | Longer times at lower temps can achieve similar results to shorter times at higher temps. |
| Surface Preparation | Creates a clean, smooth interface for effective atomic bonding. | Critical for success; surfaces must be free of contaminants and oxides. |
Achieve Parent-Metal Strength with Your Critical Components
Diffusion bonding is a sophisticated process requiring precise control over temperature, pressure, and atmosphere to create high-integrity, near-invisible joints. Whether you are working with titanium alloys, dissimilar materials, or need to preserve sensitive microstructures, the right equipment and expertise are paramount.
KINTEK specializes in advanced thermal processing solutions for laboratory and industrial applications. Our expertise in controlled atmosphere and vacuum furnace technology can help you optimize your diffusion bonding parameters for superior results.
Ready to enhance your joining capabilities? Contact our experts today to discuss how we can support your specific material and performance requirements with reliable lab equipment and consumables.
Visual Guide

Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
- Warm Isostatic Press for Solid State Battery Research
- Warm Isostatic Press WIP Workstation 300Mpa for High Pressure Applications
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
People Also Ask
- What advantages does hot pressing sintering equipment provide for NASICON? Achieve 100% Dense Solid Electrolyte Plates
- What are the advantages of using a vacuum hot press sintering furnace? Achieve 99.1% Density in CuW30 Composites
- What role does a high-temperature hot press play in the sintering of NITE-SiC? Optimize Your Densification Process
- What conditions does a Vacuum Hot Pressing Furnace provide for Copper-MoS2-Mo composites? Achieve Peak Densification
- How does the uniaxial pressing function of a vacuum hot press furnace influence the microstructure of ZrC-SiC ceramics?



















