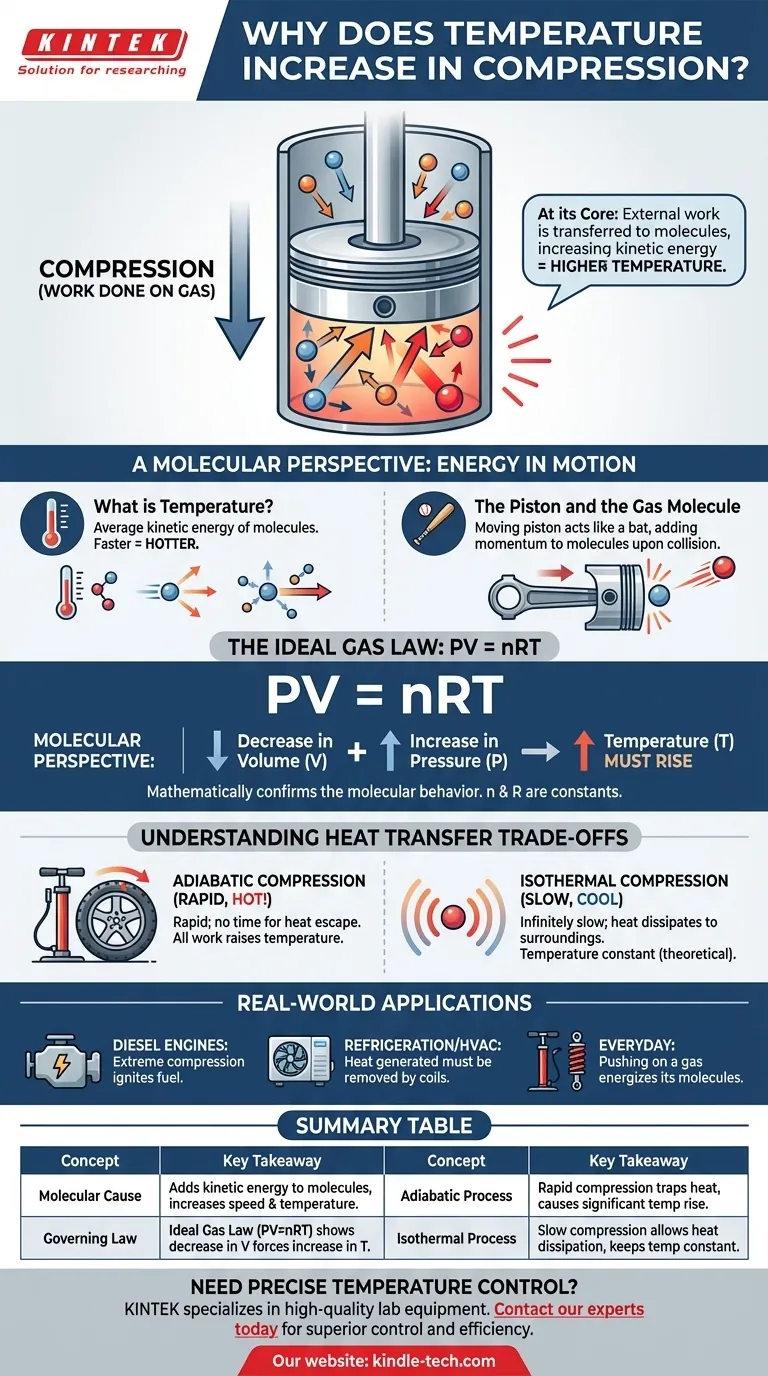
At its core, compressing a gas increases its temperature because you are performing work on it. This external work is transferred directly to the gas molecules, increasing their kinetic energy. We measure this increase in the average kinetic energy of the molecules as a rise in temperature.
The act of compression is not merely a reduction of volume; it is an injection of energy. The force you apply to compress the gas adds energy to its molecules, making them move faster, which we perceive as heat.

A Molecular Perspective: Energy in Motion
To truly understand compressional heating, we must think about what's happening at the scale of individual molecules. The temperature of a gas is simply a measure of the average motion of its countless particles.
What is Temperature, Really?
Temperature is a macroscopic measurement of the average kinetic energy of the molecules in a system. Faster-moving molecules possess more kinetic energy, resulting in a higher temperature. Slower-moving molecules mean a lower temperature.
The Act of Compression
Imagine a gas contained within a cylinder with a movable piston. To compress the gas, you must physically push the piston inward, applying force over a distance. This action is the scientific definition of work.
The Piston and the Gas Molecule
Think of the moving piston as a bat and a gas molecule as a ball. When a molecule collides with a stationary piston, it bounces off with the same speed. However, when it collides with a piston that is moving inward, the piston adds momentum and energy to the molecule, causing it to rebound with greater speed.
The Collective Effect
This energy transfer happens billions of times per second with all the molecules striking the piston's face. Each collision adds a tiny bit of energy. The cumulative result is a significant increase in the average speed—and thus the average kinetic energy—of the entire population of molecules, causing the gas's temperature to rise.
The Role of the Ideal Gas Law
This molecular behavior is described mathematically by fundamental physical laws, most simply by the Ideal Gas Law. It provides a high-level confirmation of what we see at the molecular level.
The Governing Equation: PV = nRT
The Ideal Gas Law relates the pressure (P), volume (V), and temperature (T) of a given amount of gas (n). The 'R' is a constant. This equation shows these properties are intrinsically linked.
How the Law Predicts the Outcome
When you compress a gas, you decrease its volume (V). The force you apply also increases its pressure (P). For the equation PV = nRT to remain balanced, an increase on the left side of the equation (from the combination of P increasing and V decreasing) must be matched by an increase on the right side. Since n and R are constant, temperature (T) must rise.
Understanding the Trade-offs: Heat Transfer
The rate of compression dramatically changes the outcome because it determines how much time the system has to interact with its surroundings.
Adiabatic Compression (No Heat Escape)
This occurs when compression is so rapid there is no time for the generated heat to escape into the environment. All the work you perform is converted directly into raising the gas's internal energy and temperature. Pumping a bicycle tire is a close real-world example; the pump gets noticeably hot.
Isothermal Compression (Perfect Heat Escape)
This is a theoretical ideal that occurs when compression is done infinitely slowly. This slow pace allows all the extra heat generated by the work to dissipate into the surroundings, keeping the temperature of the gas constant. While not practically achievable, it's a critical concept for thermodynamic analysis.
The Real-World Scenario
Nearly all real-world processes fall between these two extremes. Some of the work done increases the internal temperature, while some of the generated heat is lost to the environment.
How to Apply This Principle
Understanding compressional heating is not just academic; it is fundamental to countless real-world applications and systems.
- If your primary focus is everyday phenomena: Remember that pushing on a gas (doing work) energizes its molecules, which is why a bike pump or shock absorber gets hot.
- If your primary focus is engine design: This principle is the very foundation of the diesel engine, which uses extreme compression to heat air enough to ignite fuel without a spark plug.
- If your primary focus is refrigeration or HVAC: The heat generated during the compression phase of a refrigerant is the waste heat that must be actively removed by the condenser coils on the back of your refrigerator or in your outdoor AC unit.
Ultimately, the link between mechanical work and thermal energy is a fundamental law of physics, turning force into heat at the molecular level.
Summary Table:
| Concept | Key Takeaway |
|---|---|
| Molecular Cause | Compression adds kinetic energy to gas molecules, increasing their speed and temperature. |
| Governing Law | The Ideal Gas Law (PV=nRT) mathematically shows a decrease in volume (V) forces an increase in temperature (T). |
| Adiabatic Process | Rapid compression (e.g., bike pump) traps heat, causing a significant temperature rise. |
| Isothermal Process | Slow, theoretical compression allows heat to dissipate, keeping temperature constant. |
| Real-World Impact | Foundational for diesel engines, refrigeration cycles, and HVAC systems. |
Need precise temperature control for your processes? The principles of thermodynamics are critical for R&D and industrial applications. KINTEK specializes in high-quality lab equipment and consumables, serving the exacting needs of laboratories. Whether you're developing new materials or optimizing thermal systems, our expertise can support your work. Contact our experts today to discuss how we can help you achieve superior control and efficiency in your lab.
Visual Guide
Related Products
- Warm Isostatic Press WIP Workstation 300Mpa for High Pressure Applications
- Double Plate Heating Press Mold for Lab
- Anti-Cracking Press Mold for Lab Use
- Single Punch Tablet Press Machine and Mass Production Rotary Tablet Punching Machine for TDP
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
People Also Ask
- How do I prepare my house for bed bug heat treatment? A Guide to Ensuring Total Elimination
- What are the advantages and disadvantages of cold working over hot working? A Guide to Choosing the Right Metal Forming Process
- Why is it necessary to perform annealing treatment in a furnace after vacuum hot pressing Lithium Niobate samples?
- What is the purpose of the wiped film evaporator? Purify Heat-Sensitive Compounds Efficiently
- What are the basic components of an IR spectrometer? A Guide to the Core Parts of FTIR Instruments
- What are the disadvantages of oil sludge? Avoid Catastrophic Engine Damage and Costly Repairs
- What is bio-oil produced by pyrolysis? A Renewable Fuel Alternative Explained
- What is the formula for thickness of coating? Accurately Calculate Dry Film Thickness (DFT)



















