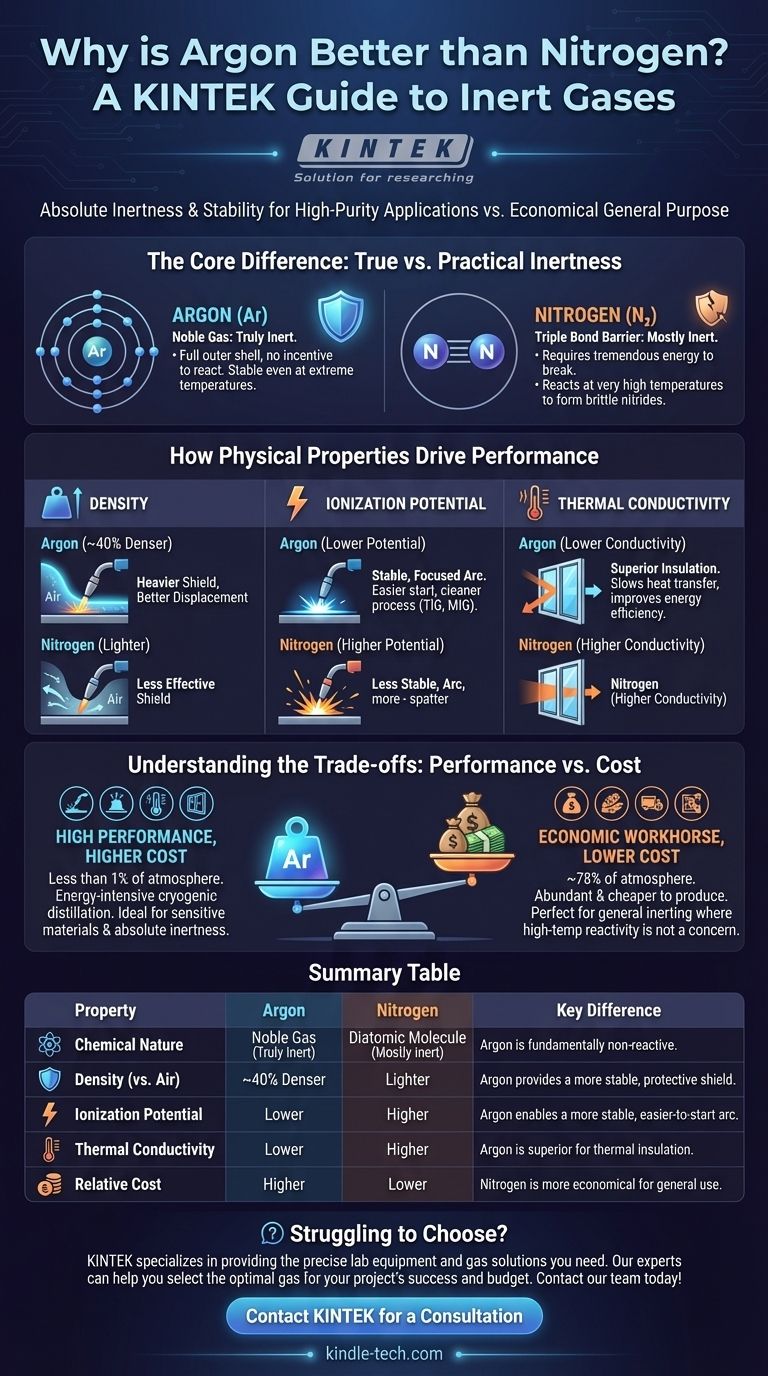
The short answer is that argon is considered "better" than nitrogen in applications that demand absolute chemical inertness and a stable environment, such as high-purity welding. This superiority stems from argon being a noble gas, making it fundamentally less reactive than nitrogen, and its greater density, which allows it to form a more effective protective shield.
The choice between argon and nitrogen is a classic engineering trade-off. Argon offers superior performance in highly sensitive applications, while nitrogen provides a perfectly suitable and more economical solution for general-purpose inerting needs. The "better" gas is the one that best matches your specific technical and budgetary requirements.

The Core Difference: True Inertness vs. Practical Inertness
The primary reason argon and nitrogen are used in similar applications is their inert, or non-reactive, nature. However, the source of their inertness is fundamentally different, which dictates their ideal use cases.
Argon: The Noble Gas Advantage
Argon is a noble gas. Its outermost electron shell is completely full, meaning it has no chemical incentive to react with other elements.
This makes argon truly inert under almost all conditions. It will not form compounds or bonds, even at the extreme temperatures found in welding arcs or in the presence of highly reactive metals.
Nitrogen: The Triple Bond Barrier
Nitrogen gas exists as a diatomic molecule (N₂), where two nitrogen atoms are joined by an exceptionally strong triple covalent bond.
This bond requires a tremendous amount of energy to break, which is why nitrogen is mostly inert in common conditions. However, at very high temperatures, this bond can break, allowing nitrogen to react with certain metals (like titanium or aluminum) to form brittle compounds called nitrides, which can compromise material integrity.
How Physical Properties Drive Performance
Beyond chemical reactivity, the physical differences between argon and nitrogen are critical in determining the right gas for the job.
Density: A Heavier Shield is a Better Shield
Argon is approximately 40% denser than nitrogen and air. This is a significant advantage in applications like welding.
When used as a shielding gas, the heavier argon effectively displaces the lighter air around the weld pool, creating a more robust and stable protective bubble. This prevents oxygen and water vapor from contaminating the molten metal. Nitrogen is less effective at this displacement.
Ionization Potential: The Key to a Stable Arc
In arc welding processes like TIG and MIG, an electric arc must be established through the shielding gas.
Argon has a lower ionization potential than nitrogen, meaning it requires less voltage to start and maintain a stable, focused arc. This results in a cleaner, more controlled welding process with less spatter, especially on sensitive metals like aluminum, titanium, and stainless steel.
Thermal Conductivity: The Impact on Insulation
Argon has lower thermal conductivity than nitrogen. This means it is a poorer conductor of heat.
This property is highly valued in manufacturing insulated double- or triple-pane windows. The space between the glass panes is filled with argon to slow the transfer of heat, improving the window's overall energy efficiency.
Understanding the Trade-offs: Performance vs. Cost
While argon has clear performance advantages in certain areas, these benefits come at a price.
Argon: High Performance, Higher Cost
Argon makes up less than 1% of the Earth's atmosphere. Separating this small fraction from air through cryogenic distillation is an energy-intensive and costly process, making pure argon significantly more expensive than nitrogen.
Nitrogen: The Economic Workhorse
Nitrogen is the most abundant gas in our atmosphere, at approximately 78%. This abundance makes it far cheaper to produce. For applications where its reactivity at high temperatures is not a concern, it is the clear economic choice.
When a Gas Mixture is the Answer
In many industrial applications, especially steel welding, a mixture of gases provides the optimal balance of performance and cost. For example, a common mixture of argon and carbon dioxide is used in MIG welding to achieve good arc stability and weld penetration on carbon steel at a lower cost than pure argon.
Making the Right Choice for Your Application
Selecting the correct gas requires you to align its properties with the specific demands of your project.
- If your primary focus is high-quality TIG or MIG welding on non-ferrous metals (aluminum, magnesium, titanium) or stainless steel: Argon is the superior choice for its absolute inertness and excellent arc stability.
- If your primary focus is bulk inerting, food packaging, or tire inflation: Nitrogen is the more cost-effective and perfectly suitable choice.
- If your primary focus is thermal insulation for high-efficiency windows: Argon's low thermal conductivity makes it the clear winner.
- If your primary focus is general-purpose MIG welding of carbon steel: An argon/CO₂ mixture often provides the best balance of cost, arc stability, and weld quality.
Ultimately, choosing the right inert gas is not about finding the single "best" one, but about precisely matching the gas's properties to your technical requirements and budget.
Summary Table:
| Property | Argon | Nitrogen | Key Difference |
|---|---|---|---|
| Chemical Nature | Noble Gas (Truly Inert) | Diatomic Molecule (Mostly Inert) | Argon is fundamentally non-reactive, even at high temperatures. |
| Density (vs. Air) | ~40% Denser | Lighter | Argon provides a more stable, protective shield. |
| Ionization Potential | Lower | Higher | Argon enables a more stable, easier-to-start welding arc. |
| Thermal Conductivity | Lower | Higher | Argon is superior for thermal insulation applications. |
| Relative Cost | Higher | Lower | Nitrogen is more economical for general-purpose use. |
Struggling to choose the right inert gas for your specific lab or production process? KINTEK specializes in providing the precise lab equipment and gas solutions you need. Our experts can help you select the optimal gas—whether it's high-purity argon for sensitive welding or cost-effective nitrogen for general inerting—to ensure your project's success, performance, and budget efficiency. Contact our team today for a personalized consultation!
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Bomb Type Probe for Steelmaking Production Process
- High Temperature Wear-Resistant Alumina Al2O3 Plate for Engineering Advanced Fine Ceramics
People Also Ask
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment
- What are the key performance characteristics and applications of platinum sheets? Unmatched Reliability for Demanding Applications
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance













