In short, Potassium Bromide (KBr) is used in IR spectroscopy because it is transparent to infrared radiation and can be easily pressed into a thin, solid disc that holds the sample. This KBr disc, or pellet, acts as a "window," allowing the spectrometer's IR beam to pass through the sample without interfering with the measurement.
The fundamental challenge in IR spectroscopy is to measure only the light absorbed by the sample, not the sample holder. KBr is an ideal material for this because it's essentially invisible to the IR beam in the most useful frequency range, ensuring the resulting spectrum is a true chemical fingerprint of the substance being analyzed.

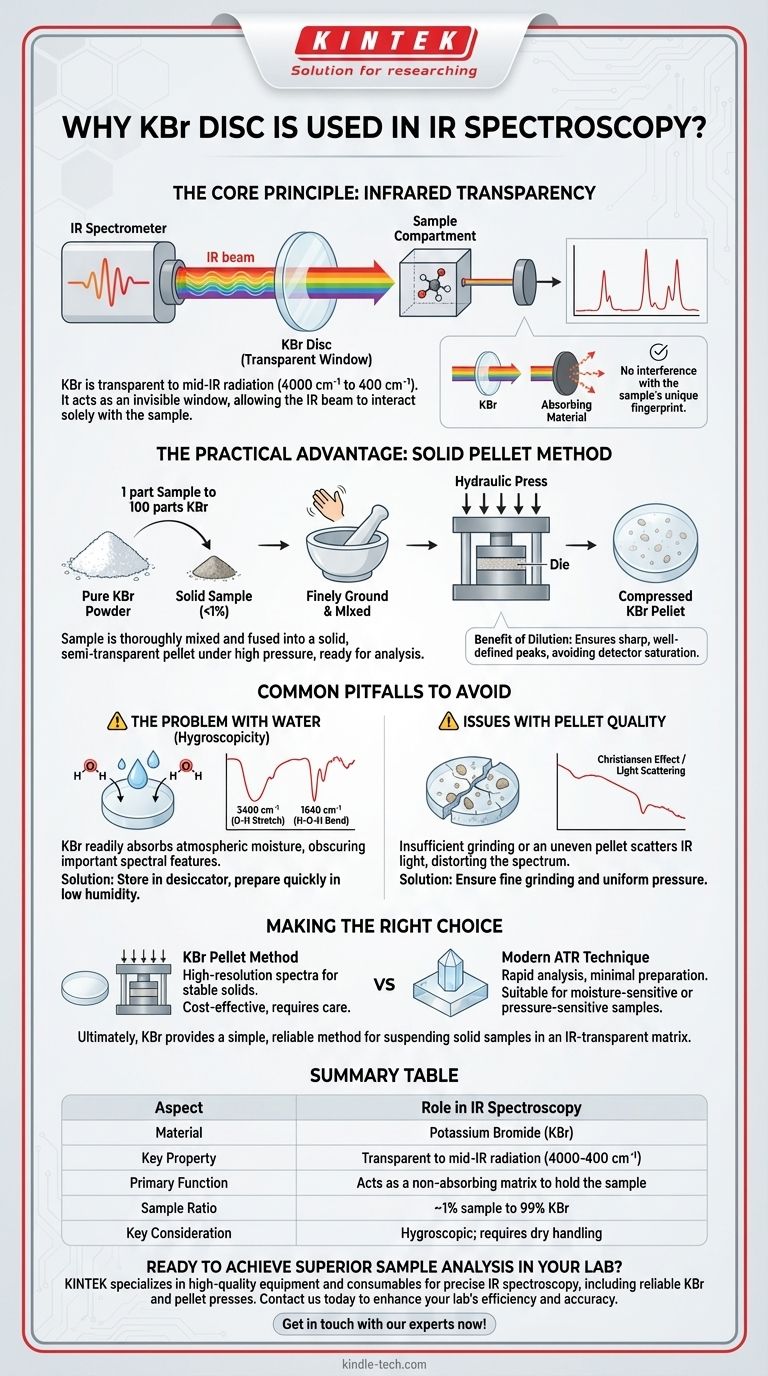
The Core Principle: Infrared Transparency
To understand the role of KBr, we must first understand the goal of an IR spectrometer. It shines a beam of infrared light through a substance and measures which frequencies of light are absorbed.
Why Transparency is Non-Negotiable
The specific frequencies absorbed by a molecule correspond to the vibrations of its chemical bonds. This pattern of absorption peaks is unique to that molecule.
If the material holding the sample also absorbed IR light, its absorption peaks would overlap with the sample's peaks. This would make it impossible to distinguish the two, rendering the final spectrum useless.
KBr's Ideal Spectral Window
Materials like KBr are alkali halides. They are simple ionic salts whose crystal lattice vibrations occur at very low frequencies, far outside the standard mid-IR range (4000 cm⁻¹ to 400 cm⁻¹) used for analysis.
Because KBr has no molecular bonds that absorb light in this critical region, it provides a perfect, clear "window" for the IR beam to interact solely with the sample.
The Practical Advantage: The Solid Pellet Method
Beyond transparency, KBr is favored for its physical properties, which allow for a simple and effective method of preparing solid samples.
Preparing the Sample Mixture
A very small amount of the solid sample is finely ground with pure, dry KBr powder. The typical ratio is about 1 part sample to 100 parts KBr.
This process thoroughly mixes the sample molecules and isolates them within the KBr matrix.
Creating the Transparent Disc
The fine powder mixture is placed into a die and compressed under immense pressure using a hydraulic press.
This high pressure causes the KBr powder to fuse into a solid, semi-transparent pellet. The sample is now trapped and evenly dispersed within this solid KBr disc, ready for analysis.
The Benefit of Dilution
The high ratio of KBr to the sample is intentional. It ensures the sample is dilute enough that its absorption peaks are sharp and well-defined, not so intense that they overwhelm the detector.
Common Pitfalls to Avoid
While effective, the KBr pellet technique requires care to yield good results. Understanding its primary limitation is crucial for accurate analysis.
The Problem with Water (Hygroscopicity)
The most significant drawback of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere.
This absorbed water will appear in the IR spectrum as two distinct, broad peaks: one around 3400 cm⁻¹ (O-H bond stretching) and another around 1640 cm⁻¹ (H-O-H bond bending). These peaks can easily obscure important features of the actual sample.
To avoid this, KBr must be stored in a desiccator or oven, and the pellet must be prepared quickly in a low-humidity environment.
Issues with Pellet Quality
If the sample and KBr are not ground finely enough, IR light can scatter off the large particles. This leads to a sloping, distorted baseline in the spectrum, a phenomenon known as the Christiansen effect.
An uneven or cracked pellet will also scatter light and produce a poor-quality spectrum.
Making the Right Choice for Your Goal
The KBr disc method is a classic technique that remains highly relevant for obtaining high-quality spectra from solid samples.
- If your primary focus is obtaining a high-resolution spectrum of a stable solid: The KBr pellet method is an excellent and cost-effective choice, provided you control for moisture and prepare the pellet carefully.
- If your sample is sensitive to moisture, pressure, or you need rapid analysis: Modern techniques like Attenuated Total Reflectance (ATR) spectroscopy, which requires little to no sample preparation, may be a more suitable alternative.
Ultimately, using KBr provides a simple and reliable way to suspend a solid sample in an IR-transparent matrix, allowing for a clear measurement of its molecular structure.
Summary Table:
| Aspect | Role in IR Spectroscopy |
|---|---|
| Material | Potassium Bromide (KBr) |
| Key Property | Transparent to mid-IR radiation (4000-400 cm⁻¹) |
| Primary Function | Acts as a non-absorbing matrix to hold the sample |
| Sample Ratio | ~1% sample to 99% KBr |
| Main Advantage | Enables high-resolution spectra of solid samples |
| Key Consideration | Hygroscopic; requires dry handling to avoid water peaks |
Ready to achieve superior sample analysis in your lab?
KINTEK specializes in providing the high-quality laboratory equipment and consumables you need for precise IR spectroscopy, including reliable KBr and pellet presses. Our products are designed to help you avoid common pitfalls like moisture contamination and ensure you get clear, accurate spectra every time.
Contact us today to discuss how our solutions can enhance your lab's efficiency and accuracy. Let KINTEK be your partner in precision.
Get in touch with our experts now!
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- Custom PTFE Teflon Parts Manufacturer Corrosion Resistant Cleaning Rack Flower Basket
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Hexagonal Boron Nitride HBN Ceramic Ring
People Also Ask
- How do you prepare samples for IR? A Guide to Solid, Liquid, and Gas Sample Prep
- What is a hydraulic floor press used for? A Versatile Tool for Industrial and Lab Applications
- What is the law behind the hydraulic press? Understanding Pascal's Law for Immense Force
- How does it affect the performance of hydraulic machines? Maximize Your ROI with Precision Engineering
- Why are KBr pellets used in FTIR? Achieve Clear, Accurate Solid Sample Analysis
- What is a KBr disc? The Key to High-Quality FTIR Spectroscopy for Solid Samples
- What is the application of press forging? Manufacturing Critical High-Strength Components
- Why KBr is used in the KBr pellet method? The Ideal Matrix for Clear IR Spectroscopy
















