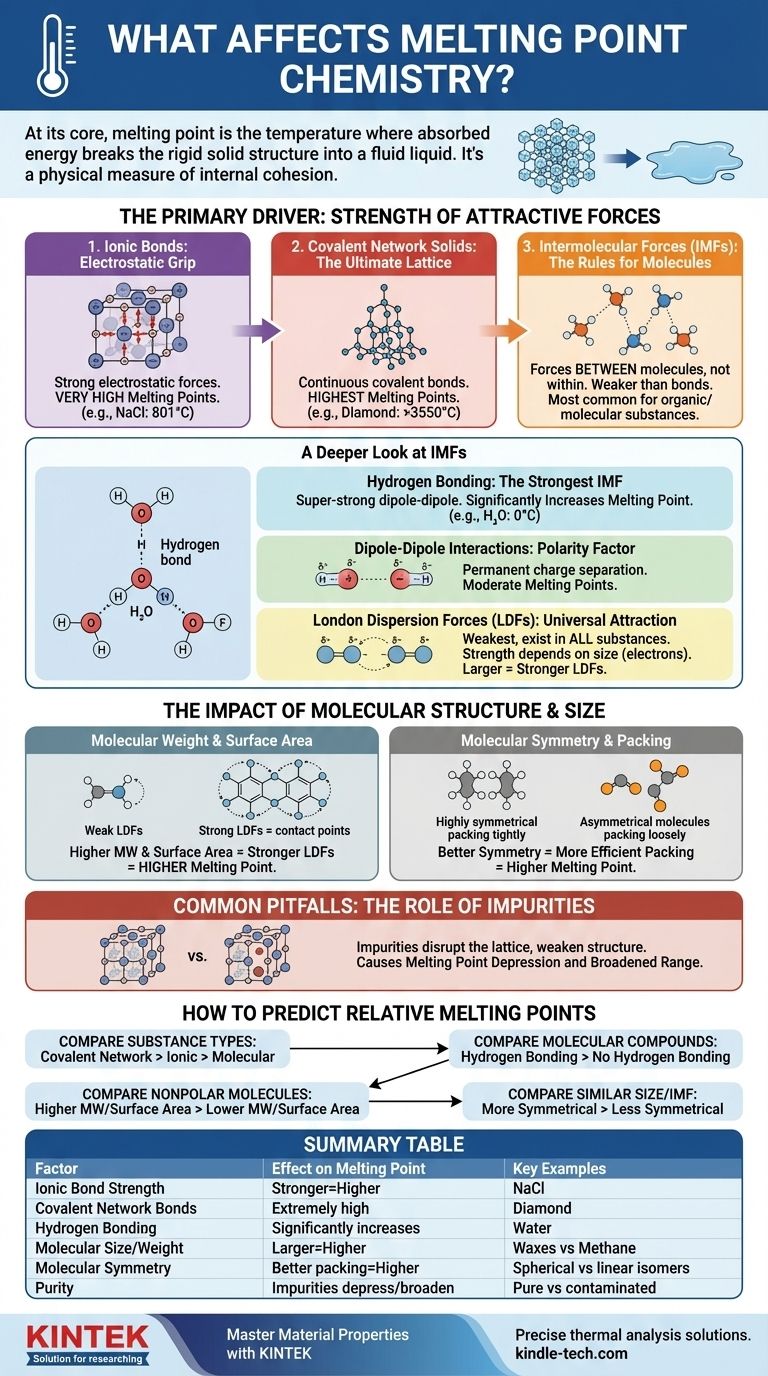
At its core, the melting point of a substance is the temperature at which it has absorbed enough energy to break the rigid, ordered structure of a solid and transition into a disordered, fluid liquid. This is determined by two primary factors: the strength of the attractive forces holding the particles (atoms, ions, or molecules) together, and the efficiency with which those particles pack into a solid crystal lattice.
The melting point isn't just a number; it's a physical measure of a substance's internal cohesion. To understand it, you must first identify the dominant force holding the particles together—be it a powerful ionic bond or a weak intermolecular attraction—and then consider how molecular size and shape refine that value.

The Primary Driver: Strength of Attractive Forces
The amount of energy needed to disrupt a solid lattice is directly proportional to the strength of the forces holding it together. These forces exist on a vast spectrum, from incredibly strong chemical bonds to much weaker intermolecular forces.
Ionic Bonds: The Electrostatic Grip
Ionic compounds, like table salt (NaCl), are held together by powerful electrostatic attractions between positive and negative ions. These forces create a very stable crystal lattice.
Overcoming these strong attractions requires a tremendous amount of thermal energy, which is why ionic compounds typically have very high melting points.
Covalent Network Solids: The Ultimate Lattice
In a covalent network solid, like diamond or quartz (SiO₂), atoms are not just attracted to each other; they are joined by a continuous network of strong covalent bonds. There are no individual molecules to separate.
To melt such a substance, you must begin to break these powerful covalent bonds. This requires more energy than any other type of interaction, giving these materials the highest melting points of all substances.
Intermolecular Forces (IMFs): The Rules for Molecules
For molecular compounds (like water, sugar, or wax), the melting point is not about breaking the covalent bonds within the molecules. It's about overcoming the weaker forces of attraction between the molecules. These are known as intermolecular forces (IMFs).
A Deeper Look at Intermolecular Forces (IMFs)
The type and strength of IMFs are the single most important factor for determining the melting point of most organic and molecular substances. They are generally much weaker than full chemical bonds.
Hydrogen Bonding: The Strongest IMF
This is a special, super-strong type of dipole-dipole interaction that occurs when hydrogen is bonded to a highly electronegative atom like nitrogen (N), oxygen (O), or fluorine (F).
The resulting attraction between molecules is significant. Water (H₂O) is a classic example; its hydrogen bonds give it a much higher melting point (0 °C) than would be expected for a molecule of its size.
Dipole-Dipole Interactions: The Polarity Factor
Polar molecules have a permanent separation of charge, creating a positive and a negative end, like tiny magnets. These molecular "poles" attract each other.
These forces are stronger than the forces between nonpolar molecules of a similar size, leading to moderate melting points.
London Dispersion Forces (LDFs): The Universal Attraction
LDFs are the weakest type of IMF and exist in all substances. They arise from the random, temporary fluctuations in electron distribution around a molecule, which creates fleeting, instantaneous dipoles.
The strength of LDFs is directly dependent on the size of the molecule (specifically, its number of electrons). Larger molecules have larger, more "sloshy" electron clouds, making them more polarizable and leading to stronger LDFs. This is why large, nonpolar molecules like wax can still be solid at room temperature.
The Impact of Molecular Structure and Size
Beyond the type of force, the specific shape and size of a molecule play a critical role in refining its melting point.
Molecular Weight and Surface Area
For molecules with the same dominant IMF (e.g., comparing two nonpolar molecules), the one with the higher molecular weight will have stronger LDFs and therefore a higher melting point. Greater surface area allows for more points of contact between molecules, also strengthening LDFs.
Molecular Symmetry and Packing
Symmetry has a profound effect. Highly symmetrical molecules can pack together more efficiently and tightly into a stable crystal lattice, like well-made LEGO bricks.
This dense, ordered arrangement requires more energy to break apart. Therefore, a more symmetrical molecule will often have a significantly higher melting point than a less symmetrical isomer, even if they have the same formula and weight.
Common Pitfalls to Avoid: The Role of Impurities
In a practical, real-world context, one of the most common factors affecting melting point is the purity of the sample.
Disruption of the Crystal Lattice
Impurities are foreign particles that do not fit neatly into the substance's crystal lattice. They introduce defects and weaken the overall structure.
Because the lattice is already disrupted, it takes less energy to break it apart, resulting in a lower melting point. This phenomenon is known as melting point depression.
A Broadened Melting Range
A pure substance typically melts over a very narrow temperature range (often less than 1 °C). The presence of impurities not only lowers the melting point but also causes the substance to melt over a wider, broader temperature range. Chemists use this characteristic to assess the purity of a synthesized compound.
How to Predict Relative Melting Points
When comparing two substances, work through this hierarchy of questions to make an accurate prediction.
- If your primary focus is comparing different substance types: A covalent network solid (diamond) will have a higher melting point than an ionic compound (salt), which will be far higher than a molecular compound (sugar).
- If your primary focus is comparing two molecular compounds: First, check for hydrogen bonding. The molecule that can form hydrogen bonds will almost always have a higher melting point than one that cannot, assuming similar size.
- If your primary focus is comparing two nonpolar molecules: The molecule with the higher molecular weight and greater surface area will have stronger London Dispersion Forces and a higher melting point.
- If your primary focus is comparing two molecules of similar size and IMF type: The more symmetrical molecule that can pack more efficiently into a crystal lattice will likely have the higher melting point.
Understanding these factors transforms the melting point from a simple data point into a powerful indicator of a substance's fundamental molecular forces and structure.
Summary Table:
| Factor | Effect on Melting Point | Key Examples |
|---|---|---|
| Ionic Bond Strength | Stronger bonds = Higher melting point | Sodium Chloride (NaCl): 801°C |
| Covalent Network Bonds | Extremely high melting points | Diamond: >3550°C |
| Hydrogen Bonding | Significantly increases melting point | Water (H₂O): 0°C |
| Molecular Size/Weight | Larger molecules = Higher melting point (stronger LDFs) | Waxes (high MW) vs. Methane (low MW) |
| Molecular Symmetry | Better packing = Higher melting point | Spherical vs. linear isomers |
| Purity | Impurities depress and broaden melting range | Pure vs. contaminated samples |
Master Material Properties with KINTEK
Understanding melting points is critical for material selection, synthesis, and quality control in the lab. Whether you're developing new compounds or analyzing material purity, having the right equipment is essential.
KINTEK specializes in high-quality laboratory equipment and consumables designed to deliver precise and reliable thermal analysis. Our products help researchers and lab professionals accurately determine melting points and understand material behavior.
Ready to enhance your lab's capabilities? Contact our experts today to find the perfect solution for your thermal analysis needs.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Lab-Scale Vacuum Induction Melting Furnace
- Vacuum Induction Melting Spinning System Arc Melting Furnace
- Heated Hydraulic Press Machine with Integrated Manual Heated Plates for Lab Use
- Non Consumable Vacuum Arc Induction Melting Furnace
People Also Ask
- Is biomass an efficient source of energy? A Deep Dive into Its Strategic Role in Renewable Power
- How accurate is the XRF analyzer? Achieve Lab-Quality Results in the Field
- What are the principles of physical vapor deposition of thin films? Master the 3-Step Process for High-Purity Coatings
- How are polymers used in the sintering process? Master Porosity and Strength with Expert Techniques
- What are the factors that affect the quality of heat treatment? Mastering Temperature, Atmosphere, and Process Control
- What are the different types of frames in compression? A Guide to I, P, and B-Frames
- What is the effect of sintering on hardness? Maximize Material Strength & Durability
- What is the process of sintering reaction? Transform Powder into Dense, High-Performance Parts





















