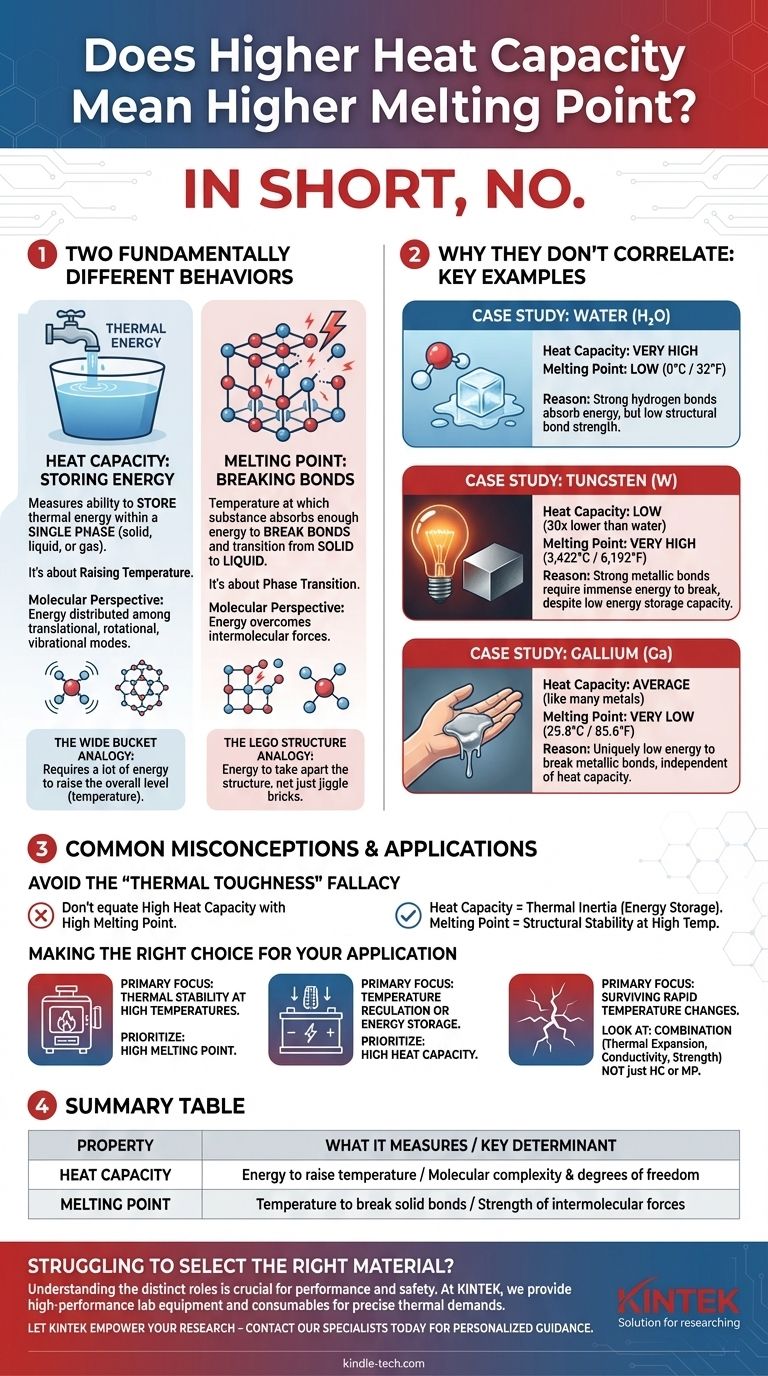
In short, no. A high heat capacity does not mean a material will have a high melting point. While both properties relate to thermal energy, they describe two fundamentally different physical behaviors, and there is no reliable correlation between them. A material like water has a very high heat capacity but a low melting point, while a metal like tungsten has a much lower heat capacity but one of the highest melting points known.
Heat capacity measures a substance's ability to store thermal energy within a single phase (solid, liquid, or gas). In contrast, a melting point is the temperature at which a substance absorbs enough energy to break the bonds holding its solid structure together and transition to a liquid. They are independent properties.

What Heat Capacity Truly Represents
It's About Storing Energy, Not Resisting Change
Specific heat capacity is the amount of energy required to raise the temperature of a specific mass of a substance by one degree. Think of it as a bucket for thermal energy.
A substance with a high heat capacity is like a very wide bucket. You have to pour a lot of energy into it to make its overall level (temperature) rise.
The Molecular Perspective: Degrees of Freedom
This energy isn't just making atoms vibrate faster in their fixed positions. The added energy is distributed among various "modes of freedom."
For molecules, this includes translational (moving), rotational (spinning), and vibrational (bonds stretching and bending) motions. Complex molecules have many vibrational modes, giving them numerous ways to store energy without increasing their average kinetic energy, which is what we measure as temperature.
What Melting Point Truly Represents
It's About Breaking Bonds, Not Storing Energy
The melting point is a single, fixed temperature where a substance undergoes a phase transition from solid to liquid.
At this temperature, any energy you add—known as the latent heat of fusion—does not raise the temperature at all. Instead, 100% of that energy is consumed to break or weaken the intermolecular bonds that hold the atoms or molecules in a rigid crystal lattice.
The Analogy: Dismantling a Structure
Imagine a structure built of LEGOs. The melting point is the point at which the builder decides to take it apart.
The energy needed to break the connections between the bricks (latent heat) is entirely different from the energy needed to make the individual bricks themselves jiggle faster (heat capacity). The strength of the connections determines the melting point.
Why They Don't Correlate: Key Examples
The disconnect between bond strength and molecular complexity leads to many examples that defy any simple correlation.
Case Study: Water (H₂O)
Water has an exceptionally high specific heat capacity. This is due to the strong hydrogen bonds between its molecules, which can absorb significant energy. However, its melting point is a familiar 0°C (32°F), which is quite low compared to many other substances.
Case Study: Tungsten (W)
Tungsten is a simple element with a specific heat capacity that is over 30 times lower than water's. Yet, its melting point is an incredibly high 3,422°C (6,192°F). This is because its strong metallic bonds require an immense amount of energy to break, locking its atoms into a solid lattice until extreme temperatures are reached.
Case Study: Gallium (Ga)
Gallium has a specific heat capacity similar to many other metals, but it has a remarkably low melting point of just 29.8°C (85.6°F). It will melt in your hand. This powerfully demonstrates that the energy required to break its metallic bonds is uniquely low, independent of its ability to store thermal energy once in a solid or liquid state.
Common Misconceptions to Avoid
The "Thermal Toughness" Fallacy
It's easy to think that a high melting point and high heat capacity both make a material "thermally tough." They don't mean the same thing. A high melting point signifies structural stability at high temperatures. A high heat capacity signifies thermal inertia, or the ability to absorb heat without a rapid temperature spike.
Confusing Heat Capacity with Latent Heat
The energy to raise the temperature to the melting point is related to heat capacity. The energy to complete the melting process at that temperature is the latent heat of fusion. These are two separate energy costs.
Overlooking Molar Heat Capacity
For many simple, solid elements, the molar heat capacity (energy per mole per degree) approaches a similar constant value at room temperature (the Dulong-Petit law). This shows that heat capacity is often more dependent on the number of atoms than the strength of the bonds between them, which is the primary factor for melting point.
Making the Right Choice for Your Application
Understanding the difference is critical for engineering and material selection. Focus on the property that solves your actual problem.
- If your primary focus is thermal stability at high temperatures: You must prioritize a high melting point. This is crucial for furnace linings, filaments, and engine components.
- If your primary focus is temperature regulation or energy storage: You must prioritize a high heat capacity. This is why water is an excellent coolant and why materials for thermal batteries are chosen for this property.
- If your primary focus is surviving rapid temperature changes (thermal shock): You must look at a combination of properties, including low thermal expansion, high thermal conductivity, and physical strength—not just heat capacity or melting point.
Choosing the right material begins with correctly identifying which thermal property directly addresses your goal.
Summary Table:
| Property | What It Measures | Key Determinant |
|---|---|---|
| Heat Capacity | Energy to raise temperature | Molecular complexity & degrees of freedom |
| Melting Point | Temperature to break solid bonds | Strength of intermolecular forces |
Struggling to select the right material for your high-temperature application? Understanding the distinct roles of heat capacity and melting point is crucial for performance and safety. At KINTEK, we specialize in providing high-performance lab equipment and consumables designed to meet the precise thermal demands of your laboratory. Whether you need materials with exceptional thermal stability or high heat capacity for energy storage, our experts can help you identify the perfect solution.
Let KINTEK empower your research — Contact our specialists today for personalized guidance on materials that will optimize your processes and results.
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
- High Purity Pure Graphite Crucible for Evaporation
- Non Consumable Vacuum Arc Induction Melting Furnace
People Also Ask
- What are the applications of graphite material? Leveraging Extreme Heat and Precision for Industrial Processes
- What temperature can graphite withstand? Unlocking Its Extreme Heat Potential
- What are the advantages of graphite furnace? Achieve High-Temperature Precision and Purity
- Why graphite is used in furnace? Achieve Superior Heat Treatment & Energy Efficiency
- What is the purpose of a graphite furnace? Achieve Extreme Temperatures for Advanced Materials













