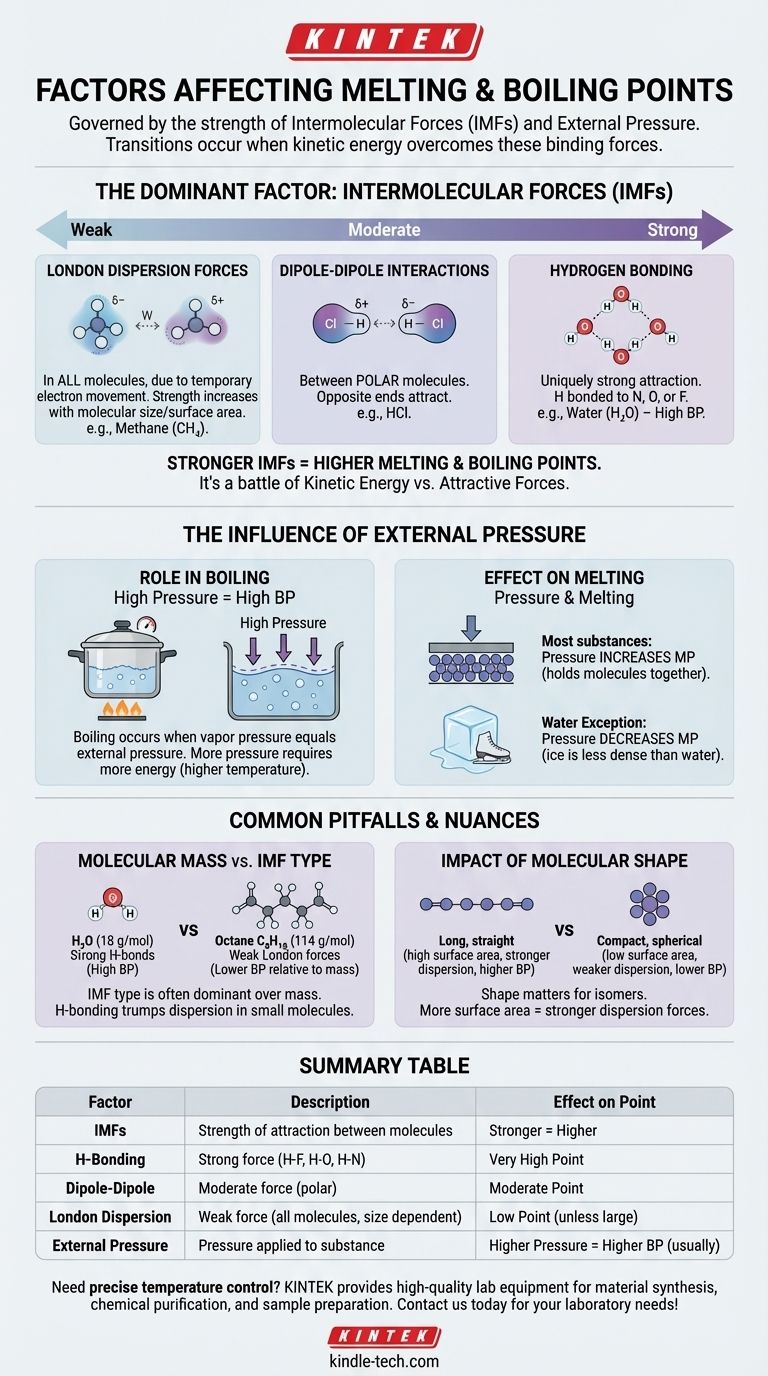
At a fundamental level, a substance's melting and boiling points are governed by two primary factors: the strength of the intermolecular forces (IMFs) holding its molecules together and the external pressure being applied to it. Essentially, these transitions happen when molecules gain enough energy to overcome the forces that bind them and the pressure that contains them.
The core takeaway is that melting and boiling are not just about temperature; they are a physical battle between a molecule's kinetic energy and the attractive forces that hold it to its neighbors. The stronger those attractions, the more energy is required to break them apart.

The Dominant Factor: Intermolecular Forces (IMFs)
The single most important factor in determining a substance's melting and boiling points is the strength of the attractions between its individual molecules. These are not the strong covalent bonds within a molecule, but the weaker forces that make molecules stick to each other.
What Are Intermolecular Forces?
Think of IMFs as the molecular equivalent of magnets. Some molecules are like powerful electromagnets, while others are like weak refrigerator magnets. Overcoming this "stickiness" requires energy in the form of heat.
The Hierarchy of Forces
Intermolecular forces vary in strength, creating a clear hierarchy. Understanding this hierarchy is key to predicting which substances will have higher or lower boiling points.
Hydrogen Bonding (Strongest) This is a uniquely strong type of attraction that occurs when hydrogen is bonded to a highly electronegative atom like nitrogen (N), oxygen (O), or fluorine (F). Water (H₂O) is the classic example, and its powerful hydrogen bonds are why it has such a remarkably high boiling point for its small size.
Dipole-Dipole Interactions (Moderate) These forces exist between polar molecules—molecules that have a permanent partial positive charge on one end and a partial negative charge on the other. These opposite ends attract each other, holding the substance together moderately well.
London Dispersion Forces (Weakest) These forces exist in all molecules, caused by the temporary, random movement of electrons that creates fleeting dipoles. Though weak individually, they become more significant as the size and surface area of the molecule increase. This is why larger molecules like octane (C₈H₁₈) are liquids at room temperature, while smaller ones like methane (CH₄) are gases.
The Influence of External Pressure
External pressure acts like a physical lid on a substance, making it harder for molecules to escape into the next phase (from solid to liquid, or liquid to gas).
The Role of Pressure in Boiling
Boiling occurs when a liquid's internal vapor pressure equals the external atmospheric pressure.
If you increase the external pressure (e.g., using a pressure cooker), you raise the boiling point because the molecules need more energy to push against that stronger external force.
Conversely, if you decrease the external pressure (e.g., by going to a high altitude), you lower the boiling point. This is why water boils at a lower temperature in Denver than it does at sea level.
The Effect of Pressure on Melting
For most substances, increasing pressure slightly increases the melting point. This is because pressure helps to hold the molecules in the rigid, tightly packed structure of a solid.
Water is a famous exception. Because solid ice is less dense than liquid water, applying pressure actually makes it easier to melt. This is why an ice skater's blade can glide over the ice.
Common Pitfalls and Nuances
Simply looking at one factor can be misleading. The interplay between forces, mass, and shape creates important nuances.
Molecular Mass vs. Intermolecular Force
While boiling points generally increase with molar mass, the type of IMF is far more dominant.
A small molecule with strong hydrogen bonds, like water (18 g/mol, boils at 100°C), will have a vastly higher boiling point than a similarly sized molecule with only weak dispersion forces, like methane (16 g/mol, boils at -161.5°C).
The Impact of Molecular Shape
For molecules with the same chemical formula (isomers), shape matters. Long, straight molecules have more surface area for contact, leading to stronger London dispersion forces and higher boiling points.
Compact, spherical molecules have less surface area and therefore weaker attractions and lower boiling points.
Making the Right Prediction
By combining these principles, you can accurately assess why different substances behave the way they do.
- If your primary focus is comparing different substances: First, identify the strongest intermolecular force present in each; this will almost always be the primary determinant of their relative boiling points.
- If your primary focus is changing the conditions of one substance: Analyze how changes in external pressure will impact the energy required for a phase transition, especially for boiling.
- If you encounter an unexpected result: Consider secondary factors like molecular shape or the unique density properties of the substance, as seen with water.
By understanding these core principles, you can move from simply memorizing melting and boiling points to truly comprehending the physical behavior of matter.
Summary Table:
| Factor | Description | Effect on Melting/Boiling Point |
|---|---|---|
| Intermolecular Forces (IMFs) | Strength of attraction between molecules. | Stronger forces = Higher point |
| Hydrogen Bonding | Strong force with H-F, H-O, H-N bonds. | Very high point |
| Dipole-Dipole | Moderate force between polar molecules. | Moderate point |
| London Dispersion | Weak force in all molecules, increases with size. | Low point (unless molecule is large) |
| External Pressure | Pressure applied to the substance. | Higher pressure = Higher boiling point (usually) |
Need precise temperature control for your lab processes? Understanding phase transitions is critical for applications like material synthesis, chemical purification, and sample preparation. At KINTEK, we specialize in high-quality lab equipment, including ovens, furnaces, and temperature control systems, designed to deliver the accuracy and reliability your research demands. Let our experts help you select the perfect equipment for your specific application. Contact us today to discuss your laboratory needs!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Induction Melting Spinning System Arc Melting Furnace
- Lab-Scale Vacuum Induction Melting Furnace
- Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press 25T 30T 50T
- Heated Hydraulic Press Machine with Integrated Manual Heated Plates for Lab Use
People Also Ask
- Why do you heat treat in a vacuum? Achieve Perfect Surface Finish and Material Integrity
- What are the parts of a vacuum furnace? A Guide to the 5 Core Systems
- What are the different types of heat treatment process for steel? Tailor Strength, Hardness & Toughness
- What are the three main heat treatments? Mastering Annealing, Hardening & Tempering
- What is a vacuum heat treatment furnace? The Ultimate Guide to Controlled Atmosphere Processing






















