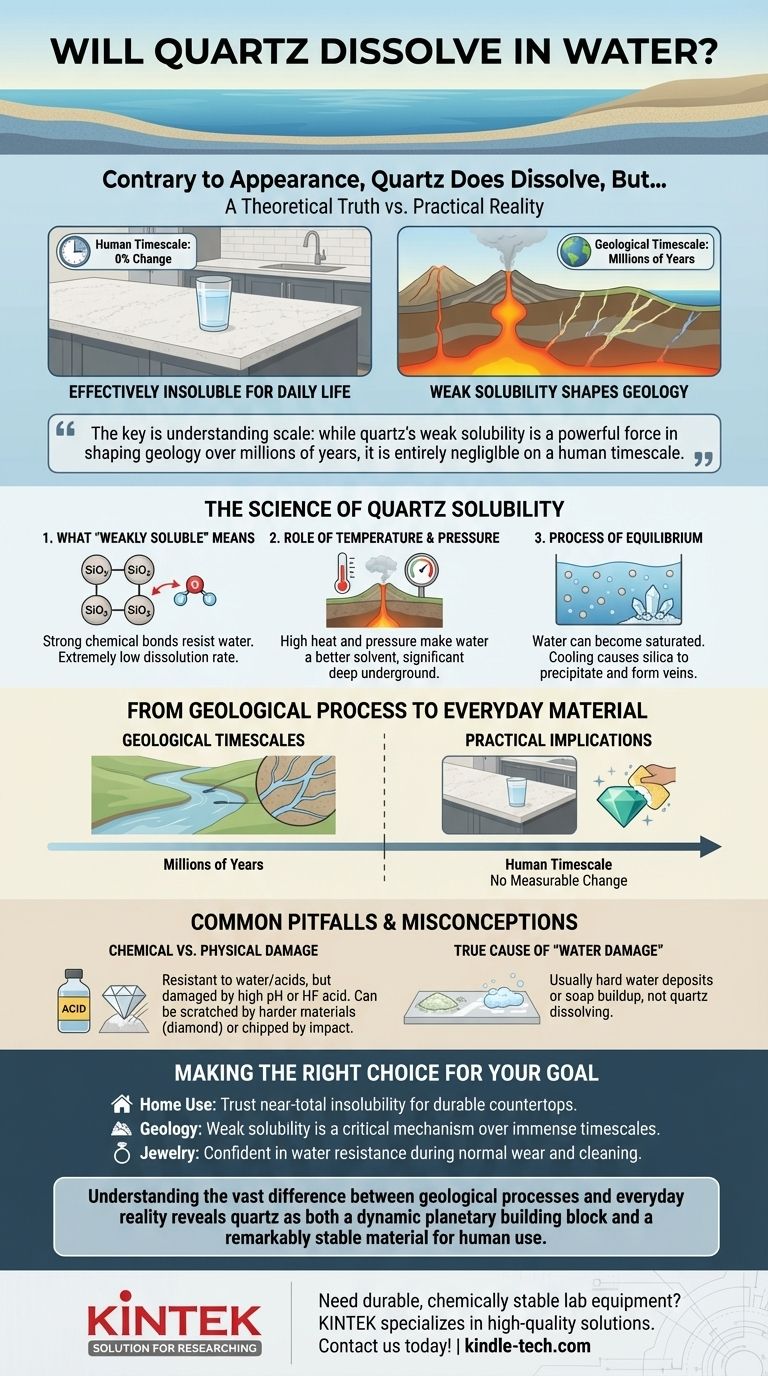
Contrary to its hard, durable appearance, quartz does dissolve in water. However, this is a theoretical truth with little practical impact in our daily lives. The process is so incredibly slow and inefficient that for all human purposes, quartz is considered effectively insoluble and chemically stable in water.
The key is understanding scale: while quartz's weak solubility is a powerful force in shaping geology over millions of years, it is entirely negligible on a human timescale. For your kitchen countertop or gemstone, water poses no chemical threat.
The Science of Quartz Solubility
To understand this apparent contradiction, we need to look at the process on a chemical level and differentiate between geological time and human time.
What "Weakly Soluble" Means
Quartz is a crystal form of silicon dioxide (SiO₂). The chemical bonds holding the silicon and oxygen atoms together are extremely strong and stable.
Water molecules can break these bonds, but they do so with immense difficulty. Unlike salt, which dissolves readily, quartz resists this chemical attack, resulting in an exceptionally low rate of dissolution.
The Role of Temperature and Pressure
The rate at which quartz dissolves is heavily influenced by its environment. Under high temperature and pressure, such as deep within the Earth's crust, water becomes a much more effective solvent.
This is why hydrothermal vents and underground water systems can carry significant amounts of dissolved silica. At the surface, under normal atmospheric conditions, this effect is minimal.
A Process of Equilibrium
Water can only hold a certain amount of dissolved silica before it becomes saturated. Once saturated, the process can reverse.
If conditions change—for example, if the silica-rich water cools or pressure drops—the dissolved silica will precipitate out of the solution, forming new quartz crystals. This is precisely how the beautiful quartz veins we see in rocks are formed over eons.
From Geological Process to Everyday Material
The reference to quartz as a "vein-filling mineral" highlights that its solubility is a fundamental geological process. But the timescale is the critical factor.
Geological Timescales
The formation of quartz veins by water dissolving and re-depositing silica happens over thousands or millions of years. It is a slow, persistent process driven by the constant flow of water through rock fissures deep underground.
Practical Implications for Daily Life
On a human timescale, the effect is nonexistent. Placing a quartz crystal in a glass of water or cleaning a quartz countertop will not cause any measurable change.
The material's durability against water is why it is prized for use in kitchens, bathrooms, and as a durable gemstone. It will not etch, fade, or weaken from exposure to water.
Common Pitfalls and Misconceptions
Understanding the nature of quartz's stability helps avoid common confusion. Its resistance to water should not be mistaken for complete indestructibility.
Chemical vs. Physical Damage
While exceptionally resistant to water and most common acids, quartz is not immune to all chemicals. Highly alkaline substances (high pH cleaners) or, more specifically, hydrofluoric acid can damage the surface.
Physically, quartz is very hard but can be scratched by materials that are harder, like topaz or diamond. It is also brittle and can be chipped or cracked by a sharp, heavy impact.
The True Cause of "Water Damage"
What is sometimes perceived as "water damage" on quartz surfaces is almost always caused by something else. Hard water can leave mineral deposits (limescale) on the surface, or soap can build up and leave a dull film. These are surface deposits, not a result of the quartz itself dissolving.
Making the Right Choice for Your Goal
Your perspective on quartz's solubility depends entirely on your context.
- If your primary focus is home use (countertops, tiles): Trust in its near-total insolubility. Quartz is one of the most durable and water-resistant materials you can choose.
- If your primary focus is geology: Recognize that the weak solubility of quartz is a critical mechanism for mineral transport and the formation of many geological features over immense timescales.
- If your primary focus is jewelry or ornamentation: Be confident that your quartz gemstone will not be harmed by water during normal wear or cleaning.
Ultimately, understanding the vast difference between geological processes and everyday reality reveals quartz as both a dynamic planetary building block and a remarkably stable material for human use.
Summary Table:
| Aspect | Practical Reality for Human Use |
|---|---|
| Solubility in Water | Effectively insoluble; no measurable change on human timescales |
| Chemical Resistance | Highly resistant to water and common acids; durable for daily use |
| Key Consideration | Weak solubility is a powerful geological force over millions of years |
Need durable, chemically stable lab equipment? KINTEK specializes in high-quality lab equipment and consumables, ensuring your laboratory operates with reliable materials that stand the test of time. Contact us today to find the perfect solutions for your laboratory needs!
Visual Guide
Related Products
- High Temperature Resistant Optical Quartz Glass Sheet
- Optical Window Glass Substrate Wafer Single Double Sided Coated K9 Quartz Sheet
- Single Punch Tablet Press Machine and Mass Production Rotary Tablet Punching Machine for TDP
- Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press 25T 30T 50T
- High Energy Planetary Ball Mill Machine for Laboratory Horizontal Tank Type
People Also Ask
- What is a vacuum tube furnace? Achieve Purity and Precision in High-Temperature Processing
- Why is a flow-controlled fixed-bed quartz reactor preferred for coal pyrolysis? Ensure High-Temperature Data Integrity
- What are the different types of tube furnace? A Guide to Horizontal, Vertical, Split, and Multi-Zone Designs
- What role does a programmable tube furnace play in IrO2/ATO catalyst synthesis? Master Stepped Deposition Today
- Why is a tube furnace required for LiCoO2 thin film treatment? Unlock Crystalline Efficiency with Controlled Oxygen
- What role does an industrial tube furnace play in a catalytic cracking experimental setup? Enhance Reaction Precision
- What are the applications of tubular furnace? Precision Heating for Research & Small-Batch Production
- What is the function of a tube furnace in the FCCVD process? Essential Catalyst for Carbon Nanotube Sheet Production



















