Introduction to Rotating Disc Electrodes
Historical Development
The concept of the rotating disk electrode (RDE) was first conceptualized by the prominent physicist Boris Levich in 1942. Levich's theoretical framework laid the groundwork for what would become a pivotal tool in the field of electrochemistry. The practical validation of this theory, however, came later with the experimental confirmation by Siver and Kabaonv in 1949. Their work demonstrated that the RDE could effectively address the limitations of earlier electrode designs, such as the static and vibrating wire electrodes.
The RDE's ability to rotate allowed for a more controlled and predictable flow of electrolyte around the electrode, which was a significant advancement. This rotational motion facilitated a uniform distribution of current density, thereby reducing the influence of the diffusion layer. As a result, the RDE became a cornerstone in electrochemical research, enabling more accurate and reproducible measurements.
The introduction of the RDE marked a turning point in the study of electrode reactions. It allowed researchers to delve deeper into the intricacies of electrochemical processes, providing insights that were previously unattainable. The RDE's design, combining the principles of electrode theory with fluid dynamics, set a new standard for electrode efficiency and reliability.
In summary, the historical development of the RDE from Levich's theoretical inception to Siver and Kabaonv's experimental validation represents a significant leap forward in the field of electrochemistry. This advancement not only overcame the shortcomings of earlier electrode designs but also opened new avenues for research and application.

Purpose and Advantages
Rotating Disc Electrodes (RDEs) serve multiple critical functions in electrochemical research, primarily focusing on enhancing the accuracy and depth of experimental results. One of their primary uses is to study the distribution of current density across the electrode surface. By rotating the disc, researchers can achieve a more uniform distribution of current, which helps in obtaining more precise measurements and insights into the electrochemical processes occurring at the electrode.
Moreover, RDEs are instrumental in minimizing the influence of the diffusion layer, a key factor that can obscure the true nature of electrode reactions. The controlled rotation of the disc effectively reduces the thickness of the diffusion layer, thereby improving the resolution of the measurements. This reduction is crucial for accurately interpreting the data, especially in complex electrochemical systems where diffusion effects can be significant.
In addition to these technical advantages, RDEs are also employed to detect and analyze electrode reaction products. This capability is particularly valuable for identifying intermediate products formed during the reaction, which can provide critical information about the reaction pathway and mechanism. By capturing these intermediates, researchers can gain a deeper understanding of the overall reaction process, which is essential for optimizing the performance of electrochemical systems.
Furthermore, RDEs are extensively used to explore complex electrode reactions. The ability to control the diffusion layer and current density distribution allows researchers to dissect intricate reaction mechanisms, which would be difficult to study using traditional static electrodes. This makes RDEs an indispensable tool in modern electrochemical research, enabling advancements in various fields such as energy storage, catalysis, and environmental science.
Fundamental Principles
Working Principle
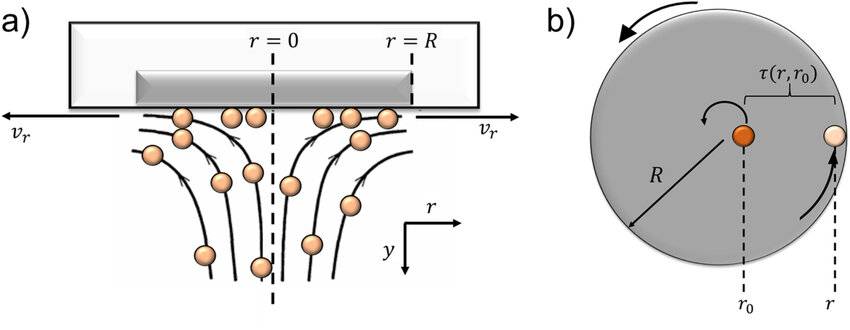
The Rotating Disc Electrode (RDE) integrates principles from both electrode theory and fluid dynamics to precisely manage the transfer of substances and current density. This integration is pivotal in creating a controlled electrochemical environment. The electrode itself is designed with a disc structure that features axial symmetry, ensuring uniform distribution of electrochemical processes across its surface.
One of the key advantages of the RDE is its minimal surface roughness, which significantly reduces irregularities that could otherwise interfere with the uniformity of the electrochemical reactions. This smoothness is crucial for maintaining consistent current density and for accurately measuring the diffusion layer thickness. The design of the electrode thus plays a critical role in the accuracy and reliability of the data collected during experiments.
In addition to its structural advantages, the RDE's operational principle leverages fluid dynamics to control the diffusion layer. By rotating the disc, the RDE creates a predictable and controllable flow of electrolyte around the electrode, which helps in maintaining a steady state of electrochemical reactions. This rotational motion ensures that the diffusion layer remains thin and uniform, further enhancing the precision of electrochemical measurements.
Structure and Design
The disc electrode is meticulously engineered to optimize its performance in electrochemical studies. Central to its design is a disc that is concentric with the rotational axis, ensuring uniform flow of the electrolyte across its surface. This axial symmetry is crucial for maintaining consistent current density distribution, which is fundamental for accurate electrochemical measurements.
To minimize interference from hydrodynamic edge effects, the electrode is encased in a thick insulating layer. This design feature effectively isolates the active disc area from the surrounding environment, preventing any potential disturbances that could arise from the fluid dynamics at the edges. Such insulation is essential for maintaining the integrity of the experimental data, as it ensures that the observed electrochemical responses are solely attributable to the active disc surface.
Additionally, the surface roughness of the electrode is kept to a minimum, significantly less than the thickness of the diffusion layer. This low surface roughness is pivotal for reducing the variability in the diffusion layer formation, thereby enhancing the reproducibility and accuracy of the measurements. By minimizing surface irregularities, the electrode facilitates a more predictable and uniform diffusion process, which is critical for the precise analysis of electrode reactions.
Applications
Electrode Reaction Studies
Rotating Disc Electrodes (RDEs) serve as powerful tools for the comprehensive analysis of electrode reactions. By leveraging the controlled hydrodynamic conditions provided by the rotating disc, researchers can detect and identify the products of electrode reactions with high precision. This capability extends to the study of intermediate products, which are crucial for understanding the detailed mechanisms of complex electrochemical processes. Additionally, RDEs enable the assessment of the stability of reaction products once they are captured on the electrode surface. This multifaceted approach not only enhances our understanding of the reaction pathways but also provides insights into the durability and behavior of these products under different experimental conditions.
Exploring Electrode Processes
The measurements obtained from rotating disc electrodes (RDEs) play a crucial role in deciphering the intricate mechanisms underlying complex electrode reactions. By providing detailed insights into the dynamics of these reactions, RDEs enable researchers to dissect the various steps involved, from the initial electron transfer to the eventual formation of products. This capability is particularly valuable in modern electrochemical research, where understanding the nuances of electrode processes is essential for advancing technology and applications.
One of the key advantages of using RDEs in these explorations is their ability to minimize the influence of the diffusion layer, thereby enhancing the accuracy and reliability of the data collected. This is achieved through the controlled rotation of the disc electrode, which ensures a steady and predictable flow of reactants to the electrode surface. As a result, RDEs are frequently employed in studies aimed at identifying and characterizing intermediate products, which are often fleeting and difficult to detect using other methods.
Moreover, the use of RDEs extends beyond mere detection; they are also instrumental in assessing the stability and reactivity of the products formed on the electrode surface. This comprehensive approach allows for a deeper understanding of the reaction pathways and the factors that influence them, contributing significantly to the broader field of electrochemistry.
Related Products
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
- Electrode Polishing Material for Electrochemical Experiments
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
Related Articles
- Advantages of the Rotating Electrode Method
- Comprehensive Guide to Rotating Disk Electrode (RDE) in Electrochemical Studies
- Introduction to Rotating Disc Electrodes and Common Electrochemical Applications
- Exploring Rotating Electrode Technology in Electrochemistry
- The Future of Electrochemical Electrodes
















