Types of Electrolytic Cells
Single-Chamber Electrolytic Cell
The single-chamber electrolytic cell is specifically designed for corrosion studies, where the primary objective is to monitor and analyze the corrosive effects on materials in a controlled electrochemical environment. Unlike other configurations, this type of cell does not separate the research electrode from the auxiliary electrode. This design choice is deliberate, as it allows for direct interaction between the electrodes, facilitating more accurate and immediate observations of corrosion phenomena.
In a single-chamber setup, the lack of physical separation between the research and auxiliary electrodes streamlines the experimental process, making it particularly suitable for studies requiring real-time data collection. This configuration minimizes potential errors that could arise from the introduction of barriers or diaphragms, which might otherwise alter the electrochemical behavior of the system.
Moreover, the single-chamber design is advantageous for its simplicity and ease of use, which is crucial for researchers who need to conduct numerous experiments in a short period. The straightforward setup reduces the complexity of the experimental apparatus, allowing scientists to focus more on the data analysis and interpretation rather than the technical intricacies of cell configuration.
In summary, the single-chamber electrolytic cell offers a practical and efficient solution for corrosion studies, providing researchers with a direct and uncomplicated means to observe and analyze electrochemical processes.

Double-Chamber Electrolytic Cell
Also known as H-type electrolytic cells, these devices are designed to separate the working electrode and the auxiliary electrode using a diaphragm. This separation is crucial to prevent any potential interference between the two electrodes, ensuring more accurate and reliable electrochemical measurements. The diaphragm, often made of porous glass, not only facilitates even current distribution but also minimizes the risk of cross-contamination, which is particularly important in sensitive experiments.
The design of double-chamber electrolytic cells is optimized for applications where maintaining the integrity of the electrochemical environment is paramount. For instance, in corrosion studies, where even minor interferences can lead to significant deviations in results, the use of a diaphragm ensures that the working electrode remains isolated from the auxiliary electrode. This isolation is achieved without compromising the efficiency of the electrochemical process, making double-chamber cells an indispensable tool in precise electrochemical testing.
In addition to their functional benefits, double-chamber electrolytic cells are also versatile in terms of the materials they can accommodate. Commonly used materials include glass and PTFE, which are chosen for their stability in various solutions. This adaptability allows researchers to use these cells in a wide range of experimental setups, from basic electrochemical studies to more complex and demanding applications.
The volume of the electrolytic cell is another critical factor in its design. The ratio of the working electrode to the solution volume must be carefully considered to meet specific test requirements. This ensures that the cell operates efficiently and that the results obtained are both accurate and reproducible. Furthermore, the ventilation of the electrolytic cell, including the design of inlet and outlet channels, is meticulously planned to manage gas dispersion and protect the integrity of the experiment.
Overall, the double-chamber electrolytic cell, with its carefully engineered design and functional components, stands as a robust solution for precise electrochemical testing, offering both reliability and versatility in a variety of research contexts.
Criteria for Electrolytic Cell Design
Material of Electrolytic Cell
The choice of material for an electrolytic cell is paramount to its effectiveness and longevity. Commonly used materials include glass and polytetrafluoroethylene (PTFE), each offering unique advantages and considerations. Glass is renowned for its transparency, allowing for visual monitoring of the electrochemical processes, and its inertness to most chemicals. However, it is susceptible to breakage and may not withstand harsh chemical environments.
PTFE, on the other hand, is highly resistant to chemical attack and offers excellent thermal stability, making it ideal for use in aggressive solutions. Its non-reactive nature ensures minimal interference with the electrolytic process. Despite these advantages, PTFE can be more challenging to fabricate into complex shapes compared to glass.
When selecting materials, it is essential to consider their stability in various solutions, including acidic, basic, and oxidative environments. The material must not only resist chemical degradation but also maintain structural integrity under the operating conditions of the electrolytic cell. This ensures accurate and reliable electrochemical testing over extended periods.
Additionally, the material's compatibility with the supporting electrolyte and the potential for contamination must be evaluated. For instance, some materials may leach impurities into the solution, affecting the accuracy of the test results. Therefore, the choice of material is a critical aspect of electrolytic cell design that directly impacts the quality and reliability of the data obtained.
Volume of the Electrolytic Cell

The volume of an electrolytic cell is a critical parameter that must be carefully considered to ensure optimal performance in electrochemical testing. The volume should be tailored to the specific needs of the experiment, taking into account the ratio of the working electrode to the solution volume. This ratio is essential for maintaining the desired electrochemical environment and ensuring that the results are accurate and reproducible.
For instance, in experiments involving corrosion studies, a larger volume may be necessary to provide a stable environment over extended periods. Conversely, in high-precision measurements, a smaller volume might be preferred to minimize the effects of diffusion and ensure rapid equilibration.
Additionally, the specific test requirements, such as the type of electrode materials used or the nature of the electrolyte, will influence the optimal volume. For example, in cells with porous glass diaphragms, the volume must be sufficient to ensure even current distribution and reduce interference from the auxiliary electrode.
In summary, the volume of an electrolytic cell should be meticulously chosen to balance the needs of the working electrode, solution volume, and specific test requirements, thereby ensuring the reliability and precision of the electrochemical measurements.
Ventilation of Electrolytic Cells
Proper ventilation in electrolytic cells is essential for maintaining optimal conditions during electrochemical processes. The primary purpose of ventilation is to facilitate the deoxygenation of the electrolyte using inert gases, such as argon or nitrogen, which helps to prevent the formation of oxygen bubbles that can interfere with the accuracy of the measurements. Additionally, effective ventilation ensures that any gases generated during the electrolysis process are safely expelled from the cell.
To achieve these goals, the design of the inlet and outlet channels must be meticulously planned. The inlet channel should be configured to evenly disperse the inert gas throughout the electrolyte, ensuring that the entire solution is deoxygenated. This even dispersion is crucial for maintaining a consistent environment within the cell, which is vital for accurate electrochemical testing.
The outlet channel, on the other hand, must be designed to efficiently remove any gases that are produced during the electrolysis process. This includes not only the inert gas used for deoxygenation but also any gases generated as byproducts of the electrochemical reactions. The outlet system should be capable of handling these gases without causing turbulence or backflow, which could compromise the integrity of the experiment.
Furthermore, the materials used in the construction of the ventilation channels must be chosen with care. They should be resistant to corrosion and capable of withstanding the chemical environment within the electrolytic cell. This ensures that the channels remain functional and do not introduce contaminants into the electrolyte.
In summary, the ventilation system of an electrolytic cell plays a critical role in ensuring the accuracy and reliability of electrochemical experiments. By carefully designing the inlet and outlet channels and selecting appropriate materials, researchers can create a stable and controlled environment that supports precise and reproducible results.
Diaphragm
In the design of double-chamber electrolytic cells, the diaphragm plays a crucial role in maintaining the integrity and accuracy of electrochemical measurements. Specifically, a porous glass separation is employed to ensure even current distribution throughout the electrolytic cell. This even distribution is vital for obtaining reliable and reproducible results in electrochemical testing.
By using porous glass, the diaphragm effectively reduces interference from the auxiliary electrode. This reduction in interference is achieved through the selective permeability of the diaphragm, which allows the passage of ions necessary for the electrochemical reaction while blocking larger particles and potential contaminants. This selective permeability ensures that the working electrode operates in a controlled and isolated environment, minimizing external influences that could skew the results.

Moreover, the diaphragm's design considerations extend to its material properties and structural integrity. The porous glass used in the diaphragm must be chemically stable and resistant to the electrolytic solutions typically used in electrochemical experiments. This stability ensures that the diaphragm does not degrade or alter the composition of the electrolyte, thereby maintaining the consistency and accuracy of the electrochemical measurements over time.
In summary, the diaphragm, particularly when constructed from porous glass, is an essential component in double-chamber electrolytic cells. Its ability to ensure even current distribution and reduce interference from the auxiliary electrode underscores its importance in achieving precise and reliable electrochemical results.
Rougin Capillary Tube
The Rougin capillary tube plays a pivotal role in electrolytic cell design, particularly in minimizing resistance between the reference and working electrodes. This critical component is essential for maintaining the accuracy and reliability of electrochemical measurements. The positioning of the Rougin capillary tube is meticulously considered to avoid potential errors that could arise from improper alignment or placement.
When designing an electrolytic cell, the Rougin capillary tube must be strategically placed to ensure optimal performance. Key factors include the distance between the reference and working electrodes, the flow rate of the electrolyte, and the overall geometry of the cell. Proper positioning helps to reduce the potential for errors such as liquid junction potentials and diffusion limitations, which can significantly impact the accuracy of the readings.
Moreover, the Rougin capillary tube is often used in conjunction with a salt bridge to further enhance the accuracy of the measurements. The salt bridge helps to reduce the liquid-connecting potential and prevent contamination, ensuring that the reference electrode remains stable and unaffected by the electrolyte solution. This combination of components ensures that the electrolytic cell operates efficiently and provides reliable data for electrochemical testing.
In summary, the Rougin capillary tube is a crucial element in electrolytic cell design, essential for minimizing resistance and ensuring accurate measurements. Its proper positioning and integration with other components, such as the salt bridge, are key considerations in achieving reliable and precise electrochemical results.
Salt Bridge
The salt bridge serves as a critical component in electrolytic cell design, connecting the reference electrode and the research electrode. This connection is essential for maintaining the integrity of electrochemical measurements by reducing the liquid-junction potential. The salt bridge acts as a mediator, allowing ions to flow between the two electrodes while preventing direct mixing of the electrolyte solutions. This separation is crucial to avoid contamination, which could otherwise skew the results of the experiment.
In practical applications, the salt bridge is often filled with a concentrated electrolyte solution, such as potassium chloride or ammonium nitrate. These electrolytes are chosen for their ability to minimize the diffusion potential at the junction between different solutions. The design considerations for the salt bridge include the type of electrolyte used, the length and diameter of the bridge, and the method of filling to ensure optimal performance.
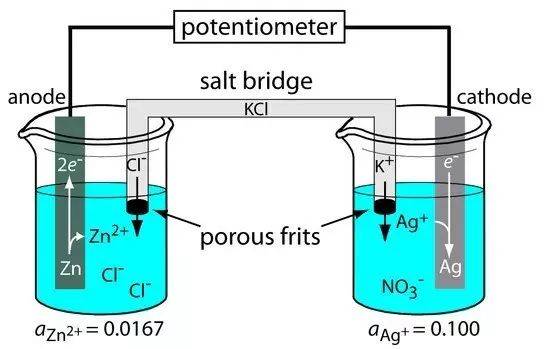
Moreover, the positioning of the salt bridge is equally important. It must be placed in such a way that it does not interfere with the flow of current between the electrodes, yet remains close enough to facilitate ion exchange. This delicate balance ensures that the salt bridge effectively reduces the liquid-junction potential without introducing additional errors or complications into the electrochemical system.
Supporting Electrolytes
Supporting electrolytes play a pivotal role in maintaining the stability and accuracy of electrochemical measurements within electrolytic cells. These electrolytes are typically added in high concentrations to achieve several critical objectives. Firstly, they effectively minimize the migration of active substances, thereby preventing any potential interference that could distort the results of electrochemical tests. This is particularly important in experiments where precise control over the chemical environment is essential.
Moreover, supporting electrolytes ensure the inertness of the electrolytic environment within the potential window. By doing so, they prevent the occurrence of side reactions that could otherwise compromise the integrity of the electrochemical data. The choice of supporting electrolyte is thus crucial, as it must not only be chemically stable but also compatible with the specific requirements of the experiment, such as the desired potential range and the nature of the active substances involved.
In practical terms, the use of high-concentration supporting electrolytes can be likened to creating a buffer zone within the electrolytic cell. This buffer zone acts as a protective barrier, safeguarding the integrity of the electrochemical process by maintaining a stable and inert environment. Consequently, the selection and addition of supporting electrolytes are not merely procedural steps but are integral to the overall success and reliability of electrochemical experiments.
Related Products
- Flat Corrosion Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- H Type Electrolytic Cell Triple Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
Related Articles
- Applications of Electrolytic Cells in Purification and Electroplating
- Advanced Electrolytic Cell Techniques for Cutting-Edge Lab Research
- Exploring the Multifunctional Electrolytic Cell Water Bath: Applications and Benefits
- Advanced Techniques in Coating Evaluation Using Electrolytic Cells
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations



















