Introduction to Glymercury Electrode
Definition and Composition
The glymercury electrode is a specialized device consisting of a solution of metallic mercury, along with its insoluble salts, Hg₂Cl₂ (calomel) and KCl (potassium chloride). The composition of this electrode is crucial as it directly influences its operational characteristics. The electrode potential of the glymercury electrode is highly sensitive to the concentration of chloride ions, a property that is extensively utilized in analytical chemistry. This sensitivity is quantified and tabulated, allowing for precise measurements in various experimental setups.
One of the notable features of the glymercury electrode is its minimal temperature coefficient of electric potential. This characteristic is particularly advantageous when the electrode is immersed in a potassium chloride solution with a concentration of 0.1 mol-dm³. In such conditions, the variation in electric potential with temperature changes is negligible, ensuring stable and reliable measurements. This stability is a key reason why the glymercury electrode is preferred in many analytical applications, especially where precise and consistent readings are essential.
Preparation and Use as a Salt Bridge
The glymercury electrode, when saturated with potassium chloride, offers a straightforward preparation process and serves as an efficient salt bridge during its operation. This ease of preparation and functionality makes it a favored choice in various analytical chemistry applications.
To prepare the glymercury electrode as a salt bridge, a solution of metallic mercury is combined with its insoluble salts, specifically Hg₂Cl₂ and KCl. The concentration of chloride ions in the solution directly influences the electrode potential, which is a critical factor in its performance. Notably, the temperature coefficient of the electric potential remains small, particularly when the potassium chloride solution is maintained at a concentration of 0.1 mol-dm.
In practical use, the glymercury electrode acts as a conduit, facilitating the movement of ions between two solutions without allowing direct mixing. This capability is crucial in maintaining the integrity of the chemical environment in each solution, thereby ensuring accurate and reliable measurements in potentiometric methods.
The glymercury electrode's role as a salt bridge is further enhanced by its compatibility with other electrodes, such as the calomel electrode, which serves as a stable reference. This pairing allows for precise determination of electrode potentials, solidifying the glymercury electrode's status as a secondary standard electrode.

Basic Information
Chinese Name
The glymercury electrode, known in Chinese as 甘汞电极 (Gān gǒng diàn jí), is a specialized tool in analytical chemistry. This electrode is composed of metallic mercury, its insoluble salts Hg₂Cl₂, and a potassium chloride (KCl) solution. The electrode's potential is sensitive to the concentration of chloride ions, making it a valuable component in various analytical procedures. Notably, the temperature coefficient of its electric potential is minimal, particularly when used with a 0.1 mol-dm potassium chloride solution, ensuring stability and reproducibility in measurements.
In the context of Chinese scientific nomenclature, the term 甘汞 (Gān gǒng) directly translates to "sweet mercury" or "glycymercury," reflecting the electrode's primary composition. This naming convention highlights the electrode's unique properties and its role in analytical chemistry, where it serves as both an indicator and a reference electrode in potentiometric methods. Its Chinese name not only signifies its chemical composition but also underscores its importance in the field, where it is often preferred for its ease of preparation and use as a salt bridge.
Applicable Temperature
The glymercury electrode is designed to function optimally within a specific temperature range, specifically below 70°C. Operating the electrode above this threshold can lead to instability in its potential value, rendering it less reliable for accurate measurements in analytical chemistry. This temperature limitation is crucial to maintain the electrode's performance and longevity, ensuring that it remains a viable option for various applications.
To understand why this temperature constraint exists, it's important to consider the components of the glymercury electrode, particularly the Hg₂Cl₂ (calomel) and KCl (potassium chloride) solutions. Above 70°C, the thermal activity can disrupt the equilibrium of these solutions, leading to fluctuations in the electrode's potential. This instability can compromise the accuracy of measurements, especially in sensitive analytical procedures.
| Temperature Range | Electrode Stability |
|---|---|
| Below 70°C | Stable and reliable |
| Above 70°C | Unstable and unreliable |
Maintaining the electrode within its recommended temperature range is essential for preserving its integrity and ensuring consistent results. This precaution ensures that the glymercury electrode remains a dependable tool in analytical chemistry, particularly in applications requiring precise and reproducible measurements.
Electrode Potential
The glymercury electrode exhibits a stable and well-documented electrode potential, which is crucial for its use in analytical chemistry. Specifically, this electrode maintains a potential of +0.2415V under standard conditions, making it a reliable reference in various electrochemical measurements.
This specific potential value is particularly significant when the electrode is used in conjunction with the standard hydrogen electrode (SHE). By comparing the potential of the glymercury electrode to that of the SHE, researchers can accurately determine the relative electrode potential of other systems. This process is essential for calibrating and standardizing electrochemical measurements, ensuring the accuracy and reproducibility of experimental results.
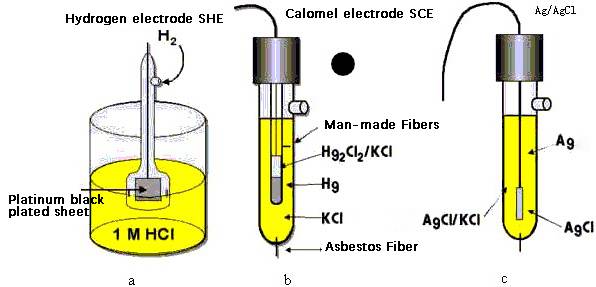
Moreover, the glymercury electrode's potential is known to be relatively insensitive to temperature changes, especially when immersed in a 0.1 mol-dm potassium chloride solution. This characteristic further enhances its utility as a reference electrode, as it minimizes potential fluctuations due to environmental variations.
In summary, the glymercury electrode's stable potential of +0.2415V, combined with its low temperature coefficient, makes it an invaluable tool in the field of analytical chemistry, particularly in potentiometric measurements and as a secondary standard electrode.
Characteristics
One of the standout features of the glymercury electrode is its small temperature coefficient of electric potential. This characteristic is particularly advantageous in analytical chemistry, where precision and stability are paramount. The temperature coefficient refers to the change in the electrode's potential with respect to temperature variations. For the glymercury electrode, this coefficient remains minimal, especially when the electrode is immersed in a potassium chloride solution with a concentration of 0.1 mol-dm. This stability ensures that the electrode potential remains relatively constant even under fluctuating temperature conditions, making it highly reliable for use in various analytical procedures.
The small temperature coefficient is a direct result of the electrode's composition, which includes metallic mercury, Hg₂Cl₂, and KCl solution. The interaction between these components helps to maintain a steady potential, which is crucial for accurate measurements in potentiometric methods. This feature not only enhances the reproducibility of the electrode but also extends its applicability across different experimental setups, where temperature control might not be perfectly maintained.
In summary, the glymercury electrode's small temperature coefficient of electric potential is a key characteristic that underscores its suitability for use as a reference electrode in analytical chemistry, where consistency and reliability are essential.
Components
The glymercury electrode is composed of three primary components: metallic mercury (Hg), mercurous chloride (Hg₂Cl₂), and a potassium chloride (KCl) solution. Each of these components plays a crucial role in the electrode's function and stability.
-
Metallic Mercury (Hg): This forms the core of the electrode and is essential for establishing the electrochemical interface. Its high density and low melting point make it suitable for maintaining a stable electrode potential.
-
Mercurous Chloride (Hg₂Cl₂): Also known as calomel, this compound is insoluble in water and acts as a buffer to stabilize the electrode potential. It ensures that the electrode maintains a consistent response to changes in chloride ion concentration.
-
Potassium Chloride (KCl) Solution: This solution serves multiple purposes. Firstly, it provides the necessary chloride ions to interact with the mercurous chloride. Secondly, it helps in the preparation of the electrode by facilitating the dissolution of other components. The concentration of KCl typically ranges from 0.1 mol-dm to saturated solutions, with the latter being more commonly used due to its ease of preparation and stability.
The combination of these components results in an electrode with a small temperature coefficient of electric potential, making it highly reliable for use in analytical chemistry, particularly in potentiometric methods.

Fields of Application
The glymercury electrode finds its primary application within the domain of analytical chemistry. This specialized electrode serves as a pivotal tool in various analytical techniques, particularly in potentiometric methods. In these methods, the glymercury electrode often functions as an indicator electrode, where its potential varies in response to changes in the concentration of specific ions in the solution being analyzed.
One of the key advantages of the glymercury electrode in analytical chemistry is its stability and reproducibility. These qualities make it an ideal candidate for use in conjunction with a reference electrode such as the calomel electrode. Together, they form a reliable electrochemical cell, enabling precise measurements of electrode potentials. This setup is crucial for applications ranging from pH measurements to the determination of various ion concentrations.
Moreover, the glymercury electrode's ability to act as a secondary standard electrode further underscores its importance in analytical chemistry. By pairing it with the standard hydrogen electrode, researchers can accurately calibrate and determine the electrode potential of the glymercury electrode, ensuring the accuracy and reliability of their analytical measurements.
In summary, the glymercury electrode's role in analytical chemistry is multifaceted, encompassing its use as an indicator electrode, its stability in potentiometric measurements, and its calibration capabilities as a secondary standard electrode. These applications highlight its indispensable nature in the field of analytical chemistry.
Basic Content in Analytical Chemistry
Indicator and Reference Electrodes
In potentiometric methods, the glymercury electrode functions as an indicator electrode, while the calomel electrode acts as a stable reference electrode. The calomel electrode is constructed from metallic mercury, calomel (Hg₂Cl₂), and potassium chloride (KCl). This configuration ensures that the calomel electrode maintains a stable and well-known electrode potential, crucial for accurate potentiometric measurements.
To achieve this stability, the calomel electrode employs a redox system with constant concentrations of its components, effectively creating a buffered system. This characteristic makes the calomel electrode an ideal reference electrode (RE) in potentiometric analysis. Unlike the indicator electrode, which responds variably to changes in the analyte, the reference electrode remains steadfast with a fixed response, providing a reliable baseline for potential measurements.
The distinction between indicator and reference electrodes is fundamental in potentiometric titrations. Indicator electrodes, such as the glymercury electrode, change their potential in response to the analyte, reflecting the concentration of the substance being measured. In contrast, reference electrodes, like the calomel electrode, maintain a constant potential, ensuring that any observed changes in potential are due to the analyte and not the measurement system itself.
Various types of indicator electrodes exist, including glass membrane, crystal membrane, and polymer membrane electrodes, each suited to different analytical needs. However, the calomel electrode remains a staple in reference electrode applications due to its reliability and simplicity in maintaining a stable potential.
Standard Hydrogen Electrode
The standard hydrogen electrode (SHE) serves as a pivotal reference in electrochemistry, providing a standardized basis for determining the relative electric potential values of other electrodes. Despite its stability, the SHE is known for its operational complexity, which often makes it less practical for routine laboratory use.
The SHE operates under standard conditions, with hydrogen gas at 1 atmosphere pressure and a concentration of 1 M hydrogen ions in solution. This setup ensures that the electrode potential remains consistent and reliable. However, the need for a constant supply of hydrogen gas and the meticulous maintenance required to maintain these conditions render the SHE cumbersome and impractical for many applications.
In contrast, other reference electrodes, such as the calomel electrode, offer a more user-friendly alternative. These electrodes, while not as universally applicable as the SHE, provide a stable and reproducible reference potential that is essential for accurate measurements in analytical chemistry.
The SHE's primary role is to establish a zero potential reference point, against which the potentials of other electrodes can be measured. This standardization is crucial for the accurate interpretation of electrochemical data and is foundational to the field of electrochemistry. Despite its limitations, the SHE remains an indispensable tool in the calibration and validation of electrochemical instruments.
Secondary Standard Electrode
The glymercury electrode, when paired with the standard hydrogen electrode, serves as a reliable tool for determining its electrode potential, thereby earning the designation of a secondary standard electrode. This pairing allows for precise measurements, which are crucial in analytical chemistry where accuracy is paramount.
The standard hydrogen electrode (SHE) is typically used as a reference to establish the relative electric potential value of other electrodes. However, SHE is known for its stability but is cumbersome to operate due to its complexity and the need for continuous hydrogen gas supply. In contrast, the glymercury electrode offers a more practical alternative.
When used in conjunction with SHE, the glymercury electrode can accurately determine its potential, making it a secondary standard. This capability is particularly valuable in analytical chemistry, where reproducibility and stability are essential for reliable results. The glymercury electrode's potential is influenced by the concentration of chloride ions, which can be precisely controlled and measured, contributing to its accuracy.
This secondary standard role of the glymercury electrode underscores its importance in potentiometric methods, where it often acts as an indicator electrode. Its ability to provide accurate potential measurements simplifies the process of calibrating other electrodes, thereby enhancing the overall precision of analytical measurements.
Uses and Applications
Reference Electrode in Potentiometry
The glymercury electrode is frequently employed as a reference electrode in potentiometry, owing to its exceptional reproducibility and stability. In potentiometric measurements, the role of a reference electrode is paramount, serving as a consistent and reliable point against which the potential of other electrodes is measured. This stability is crucial for accurate readings, as any fluctuation in the reference electrode's potential can lead to erroneous results.
To ensure this stability, reference electrodes are designed to maintain a constant potential, ideally on an absolute scale. This is achieved through two key characteristics: minimal current flow and being "well-poised." A well-poised electrode maintains its potential even when subjected to minor current flows, ensuring that its reference value remains unaffected.
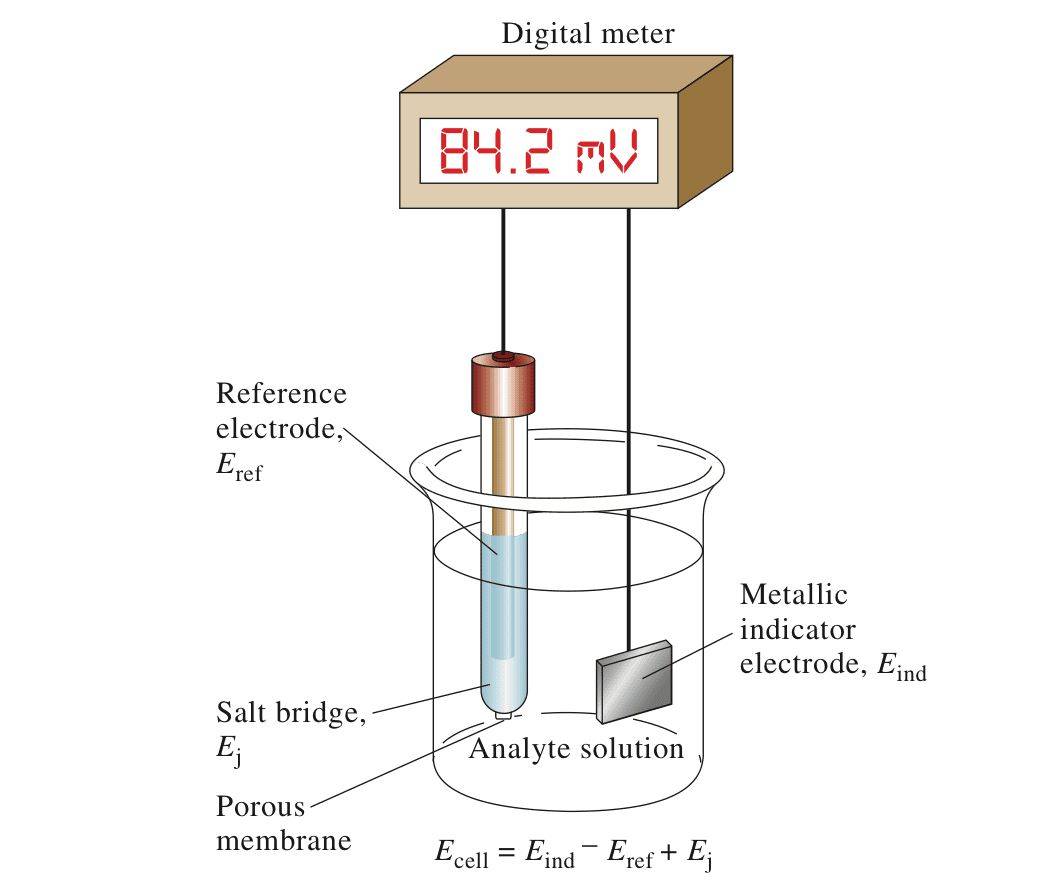
While there are several types of reference electrodes, some of the most commonly used and commercially available ones include the silver/silver chloride, saturated calomel, mercury/mercury (mercurous) oxide, mercury/mercury sulfate, and copper/copper sulfate electrodes. These electrodes are favored for their reliability and ease of use in various analytical applications.
In potentiometric titrations, the distinction between indicator and reference electrodes is critical. The indicator electrode responds to changes in the analyte, reflecting the concentration variations, while the reference electrode remains stable, providing a fixed potential. This dual setup ensures that the potential measurements are both accurate and reproducible, making the glymercury electrode an ideal choice for these applications.
Electrode Reaction and Symbols
The electrode reaction for the glymercury electrode is a fundamental process that involves the reduction of mercurous chloride (Hg₂Cl₂) to elemental mercury (Hg) and chloride ions (Cl⁻). This reaction can be represented as:
Hg₂Cl₂ + 2e⁻ → 2Hg + 2Cl⁻
This electrochemical process is crucial for the electrode's function as a reference in analytical chemistry. The symbols used to describe the glymercury electrode in a standard notation are:
Pt | Hg(l) | Hg₂Cl₂(s) | KCl(saturated)
Here, the symbols denote:
- Pt: The inert platinum wire that acts as the current collector.
- Hg(l): The liquid mercury, which is the active metal in the electrode.
- Hg₂Cl₂(s): The solid mercurous chloride, which is in equilibrium with the mercury.
- KCl(saturated): The saturated potassium chloride solution, which provides the chloride ions necessary for the electrode reaction.
This notation is essential for accurately representing the electrode's composition and the conditions under which it operates, ensuring consistency and reproducibility in experimental setups.
Temperature Limitations
The calomel electrode, while highly reliable at standard laboratory temperatures, exhibits notable instability when subjected to elevated heat. Specifically, its potential value becomes erratic above 70°C, rendering it unsuitable for precise measurements in such conditions. Furthermore, prolonged exposure to temperatures exceeding 100°C can significantly shorten the electrode's operational lifespan. Consequently, it is imperative to restrict the use of the calomel electrode to temperatures below 70°C to ensure both accuracy and longevity.
In practical applications, this limitation necessitates careful consideration when selecting the appropriate electrode for various experimental setups. For instance, in high-temperature analytical chemistry, alternative reference electrodes with broader temperature tolerances may be preferred. This ensures that the integrity of the data collected remains uncompromised by the inherent temperature constraints of the calomel electrode.
Types of Electrodes
Classification of Electrodes
Electrodes can be broadly classified into several categories based on their composition and the nature of the electrochemical reactions they facilitate. These categories include metal-metal ion electrodes, gas-ion electrodes, metal-metal insoluble salt electrodes, and redox electrodes. Each type has distinct characteristics and applications in electrochemistry.
-
Metal-Metal Ion Electrodes: These electrodes consist of a metal immersed in a solution containing its own ions. The potential of such electrodes is determined by the metal ion concentration in the solution.
-
Gas-Ion Electrodes: These electrodes involve a gas (such as hydrogen or chlorine) in equilibrium with its ions in solution. A typical example is the standard hydrogen electrode (SHE), which is used as a universal reference electrode.
-
Metal-Metal Insoluble Salt Electrodes: This category includes electrodes where a metal is in contact with an insoluble salt of the metal and a solution containing the anion of the salt. The glymercury electrode, which consists of metallic mercury in contact with its insoluble salt Hg₂Cl₂ and a potassium chloride solution, falls under this classification. The electrode potential of the glymercury electrode is influenced by the concentration of chloride ions and is known for its stability and reproducibility, making it a popular choice as a reference electrode in potentiometry.
-
Redox Electrodes: These electrodes involve a redox couple in solution, where the redox reaction occurs at an inert electrode material like platinum. The potential of a redox electrode is determined by the ratio of the oxidized to reduced species in the solution.
The glymercury electrode, with its unique composition and stable potential, is particularly significant in analytical chemistry, where it serves as a reliable reference electrode. Its classification as a metal-metal insoluble salt electrode underscores its specialized role in electrochemical measurements and its compatibility with various analytical techniques.

Other Typical Electrodes
In the realm of metal-metal insoluble salt electrodes, the silver-silver chloride electrode stands out as a notable counterpart to the glymercury electrode. This electrode is widely used in various analytical chemistry applications due to its stability and reproducibility.
Composition and Functionality
The silver-silver chloride electrode consists of a silver wire coated with a thin layer of silver chloride (AgCl), which is immersed in a potassium chloride (KCl) solution. This setup ensures a stable electrode potential, making it an excellent choice for reference electrodes in potentiometric measurements.
| Component | Role |
|---|---|
| Silver Wire | Provides the conductive surface for the electrode reaction. |
| Silver Chloride (AgCl) | Forms a stable and insoluble layer, contributing to the electrode's stability. |
| Potassium Chloride (KCl) | Acts as the electrolyte, facilitating the ionic conduction. |
Electrode Reaction
The electrode reaction for the silver-silver chloride electrode can be represented as:
[ \text{AgCl} + \text{e}^- \rightarrow \text{Ag} + \text{Cl}^- ]
This reaction highlights the transfer of electrons from the silver chloride to the silver wire, maintaining a constant potential.
Applications
The silver-silver chloride electrode is frequently employed in:
- Potentiometric Measurements: As a reliable reference electrode due to its stable potential.
- Environmental Monitoring: For accurate pH and ion concentration measurements in aqueous solutions.
- Medical and Biological Applications: In devices requiring precise and stable electrical potentials.
In summary, the silver-silver chloride electrode, alongside the glymercury electrode, plays a crucial role in the metal-metal insoluble salt electrode category, offering robust solutions for various analytical chemistry needs.
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Glassy Carbon Electrochemical Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
Related Articles
- AgAgCl Reference Electrode Working Principle and Applications
- How to Make Your Own Ag/AgCl Reference Electrode for Electrochemical Experiments
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
- Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria
- How to Choose the Right Reference Electrode for Your Application

















