In the laboratory, the greatest enemy is not lack of skill, but the invisible variable.
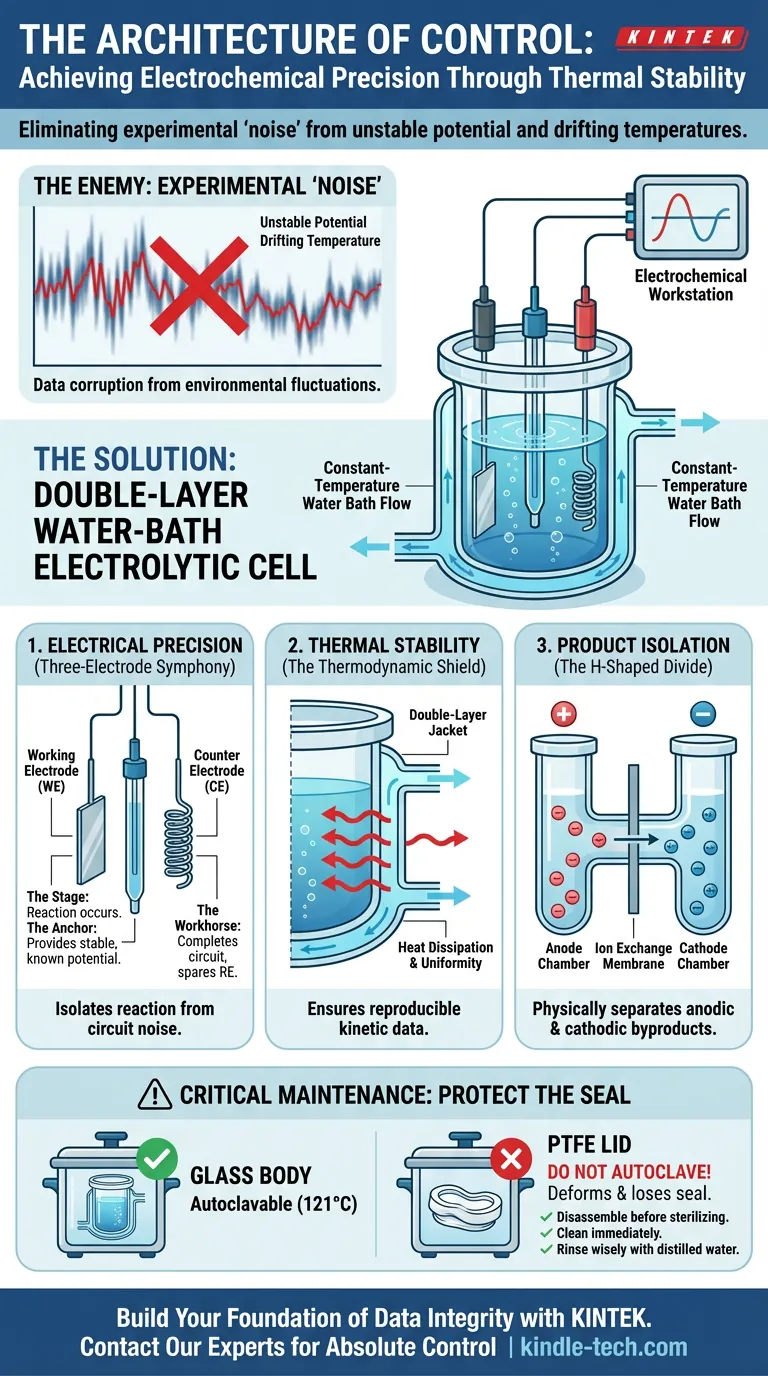
We often think of experiments as binary: they work, or they fail. But between success and failure lies the dangerous grey zone of "noise"—data that looks correct but is secretly corrupted by environmental fluctuations.
In electrochemistry, that noise usually comes from two places: unstable electrical potential and drifting temperatures.
The Double-Layer Water-Bath Electrolytic Cell is not merely a glass vessel. It is a system engineered to eliminate these two sources of chaos. It is a structure designed for control.
The Three-Electrode Symphony
To understand the system, you must understand the hierarchy of its parts. A standard setup is managed by an electrochemical workstation, but the physical reality happens within the cell using a three-electrode configuration.
Each component plays a specific psychological and physical role in the experiment:
- The Working Electrode (WE): The stage. This is where the reaction of interest actually occurs.
- The Reference Electrode (RE): The anchor. It provides a stable, known potential. It does not participate; it observes. Without it, your voltage measurements are floating relative to nothing.
- The Counter Electrode (CE): The workhorse. Also known as the auxiliary electrode, it completes the circuit, allowing current to flow without disturbing the delicate peace of the reference electrode.
When these three work in unison, you achieve electrochemical precision. You isolate the reaction you want to see from the interference you want to ignore.
The Thermodynamic Shield
However, electrical precision is useless if the thermodynamics are wild.
Electrochemical reactions are inherently sensitive to heat. They can generate their own heat (exothermic), or they can be swayed by the air conditioning kicking on in the lab. A shift of a few degrees can alter reaction kinetics, diffusivity, and equilibrium potentials.
This is why the Double-Layer Design is critical.
The cell features a "jacket"—a double-wall construction that envelops the inner chamber. By pumping a liquid from a constant-temperature water bath through this jacket, you create a thermal shield.
This serves two functions:
- Heat Dissipation: It removes excess heat generated by electrolysis.
- Uniformity: It ensures the temperature is identical at the center of the solution and at the glass wall.
The result is data that is reproducible, not just lucky.
The H-Shaped Divide
Sometimes, control requires separation.
Many advanced cells utilize an H-shaped structure. This design physically divides the anode and cathode chambers, usually separated by an ion exchange membrane.
The membrane is the negotiator. It allows necessary ions to pass through to maintain charge neutrality, but it prevents the products generated at the anode from mixing with those at the cathode.
If your goal is to study half-reactions in isolation, this structural separation is non-negotiable.
The Fragility of Hardware
There is a paradox in high-precision equipment: the more precise it is, the more specific its care must be.
We often assume that laboratory glass is invincible to heat. While the borosilicate glass body of the cell can withstand autoclaving at 121°C, the system as a whole cannot.
The weak link is the PTFE (Teflon) lid.
PTFE has a different coefficient of thermal expansion than glass. If you autoclave the assembled cell, the lid will expand, deform, and lose its ability to seal. Once the seal is gone, the control is gone.
The Maintenance Protocol
To maintain the integrity of your system, adopt a mindset of immediate action:
- Disassemble before sterilizing: Only the glass goes in the autoclave.
- Clean immediately: Residues left to dry become contaminants for the next user.
- Rinse wisely: Use distilled water. If using acids or bases for deep cleaning, verify they won't corrode the specific electrode materials.
Selecting the Right Tool for the Truth
Your choice of equipment should mirror your experimental questions. The double-layer cell is versatile, but it shines when aligned with specific goals.
| If your focus is... | The critical feature is... | Why? |
|---|---|---|
| High-Accuracy Measurement | Three-Electrode System | Isolates the reaction voltage from circuit noise. |
| Temperature Effects | Water Bath Jacket | Allows you to systematically vary 'T' as a controlled variable. |
| Product Isolation | H-Shaped Structure | physically separates anodic and cathodic byproducts. |
Science is the pursuit of removing uncertainty. The right equipment doesn't just hold your chemicals; it holds your variables constant.
At KINTEK, we understand that an electrolytic cell is the foundation of your data integrity. We specialize in providing the high-quality lab equipment and consumables necessary to build that foundation.
Do not let thermal drift or electrical noise dictate your results. Contact Our Experts to discuss how our systems can bring absolute control to your laboratory.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Electrode Fixture for Electrochemical Experiments
- Optical Water Bath Electrolytic Electrochemical Cell
Related Articles
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells
- Handheld Coating Thickness Gauges: Accurate Measurement for Electroplating and Industrial Coatings
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells




















