The Hidden Variable in Your Data
There is a distinct psychological trap in laboratory work. We tend to obsess over the variables we can see—the voltage settings, the purity of the reagents, the temperature controls.
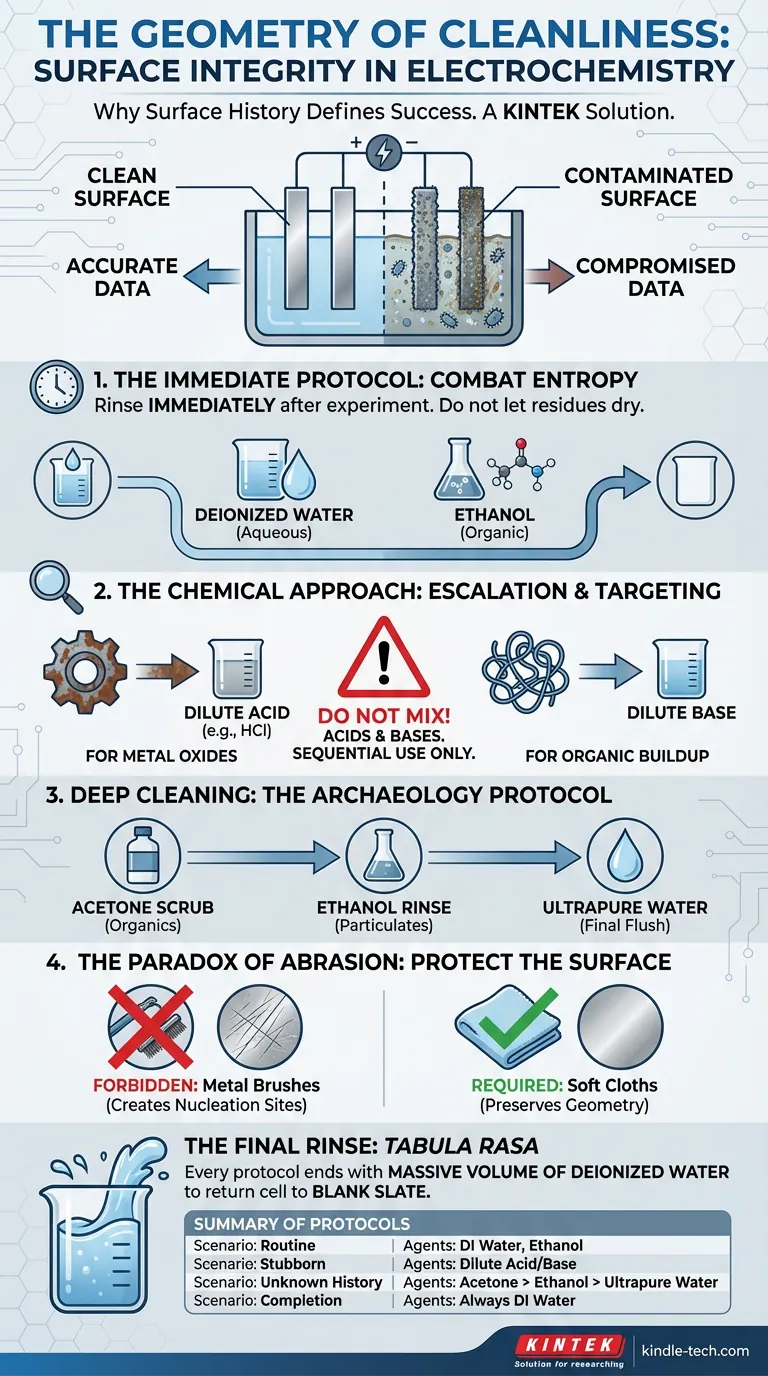
But the most significant source of error is often the one we overlook because it is invisible to the naked eye: surface history.
In electrochemistry, the reaction vessel is not a passive container. It is a stage. If that stage is cluttered with the ghosts of previous experiments—microscopic oxides or dried electrolyte salts—the performance of the current actors will be compromised.
Maintaining an electrolytic cell is not a chore; it is an engineering discipline. It requires a shift in mindset from "washing up" to "surface restoration."
Here is how to maintain the integrity of your equipment, ensuring that your data reflects the chemistry, not the contamination.
The Entropy of a Dried Surface
The most critical moment in the life of an electrolytic cell is the five minutes immediately following an experiment.
When a reaction finishes, the clock starts ticking. If you walk away to analyze data or grab lunch, the liquid residues begin to evaporate. As they dry, dissolved solids crystallize. Worse, they can react chemically with the electrode surface, forming hard, insulating layers.
Once these residues adhere, the energy required to remove them increases exponentially.
The Immediate Protocol
To combat this entropy, the rule is simple: rinse immediately.
Do not wait. As soon as the experiment concludes, flush the vessel and electrodes.
- Deionized Water: For standard aqueous solutions.
- Ethanol: For organic residues that water cannot displace.
This single act preserves the baseline state of the cell. It prevents the formation of the stubborn deposits that necessitate harsh chemical interventions later.
Escalation: The Chemical Approach
Ideally, a water rinse is enough. Realistically, it often isn’t.
When you encounter visible oxides (rust) or persistent inorganic deposits, you must escalate from physical rinsing to chemical targeting. This is where the "engineer's romance" meets practical chemistry: you must match the solvent to the solute.
Matching the Cleaner to the Contaminant
You cannot force a deposit off a surface; you must persuade it to leave.
- For Metal Oxides: Use a dilute acid (such as hydrochloric acid). The acid reacts with the oxide layer, dissolving it back into solution without harming the base glass or metal (if chosen correctly).
- For Organic Buildup: A dilute base is often more effective.
The "Do Not Mix" Rule
Chemistry is powerful, but it is indifferent to your safety. A common error in aggressive cleaning is the assumption that if one cleaner is good, two are better.
Never mix acid and alkaline cleaning agents.
Combining nitric acid (HNO₃) with sodium hydroxide (NaOH) does not create a super-cleaner; it creates a violent exothermic reaction. It endangers the scientist and shatters the equipment.
The protocol is sequential, never simultaneous: Clean with one. Rinse thoroughly. Then, and only then, use the other.
The Archaeology of Unknown Equipment
Sometimes, you inherit a cell. Perhaps it has been sitting in a cupboard for months, or perhaps you bought it used. Its history is unknown.
In these cases, you are performing an excavation. You need a "Deep Clean" protocol to reset the timeline of the device to zero.
The Trinity of Deep Cleaning:
- Acetone Scrub: Attacks organic residues and oils on the inner walls.
- Ethanol Rinse: Removes the acetone and any lingering particulates.
- Ultrapure Water: The final flush to remove all solvent traces.
The Paradox of Abrasion
There is a temptation, when faced with a stubborn spot, to use force. This is a mistake.
Glass and polished electrode surfaces rely on smoothness for their function. A scratch is not just a cosmetic defect; it is a nucleation site. It is a trench where bacteria, oxides, and ions can hide, protected from future cleaning efforts.
The Golden Rules of Physical Cleaning:
- Forbidden: Metal brushes. They destroy the geometry of the surface.
- Required: Soft cloths or non-abrasive brushes.
We must protect the equipment from our own desire to clean it too aggressively.
The Final Rinse: Tabula Rasa
The cleaning agent is, by definition, a contaminant to your next experiment.
If you clean with acid and leave a trace of it behind, you have just introduced a new variable into your next reaction. The cleaning process is not complete until the cleaning agent itself is gone.
Every protocol must end with a massive volume of deionized water. This returns the cell to a state of Tabula Rasa—a blank slate.
Summary of Protocols
Different scenarios require different levels of intervention. Use this guide to determine your approach.
| Scenario | Goal | Recommended Agents |
|---|---|---|
| Routine Maintenance | Remove bulk residue immediately. | Deionized Water, Ethanol |
| Stubborn Deposits | Target specific oxides or buildup. | Dilute Acids (e.g., HCl), Dilute Bases |
| Unknown History | Reset the cell surface completely. | Acetone $\rightarrow$ Ethanol $\rightarrow$ Ultrapure Water |
| Completion | Eliminate cleaning agents. | Always Deionized Water |
Precision Demands Partners
At KINTEK, we view laboratory equipment not as mere consumables, but as precision instruments that drive scientific truth.
We understand that an experiment is only as good as the integrity of the cell it is performed in. That is why we manufacture our electrolytic cells and consumables to withstand the rigors of both complex reactions and the necessary cleaning protocols.
Do not let surface noise drown out your data.
Visual Guide
Related Products
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Electric Lab Cold Isostatic Press CIP Machine for Cold Isostatic Pressing
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
Related Articles
- The Thermodynamic Paradox: Balancing Precision and Safety in Electrolytic Cells
- Understanding Electrodes and Electrochemical Cells
- Applications of Electrolytic Cells in Purification and Electroplating
- Applications of H-Type Electrolytic Cell in Metal Extraction
- Advanced Techniques in Coating Evaluation Using Electrolytic Cells




















