In the laboratory, we tend to obsess over the invisible variables. We worry about potential shifts, reaction kinetics, and the purity of our reagents.
But often, the success of an electrochemical experiment is determined by something far more mundane: the physical interface.
Specifically, the holes in the lid of your electrolytic cell.
These apertures are the gatekeepers. They determine what enters the system (probes, gases) and what stays out (oxygen, contaminants). Understanding the standard specifications—specifically 6.2mm and 3.2mm—is not just about hardware compatibility.
It is about understanding the limits of your experimental control.
The Architecture of the Lid
The industry has settled on a specific geometry to create order out of chaos. While designs vary, the diameters are rarely arbitrary. They are designed to answer two distinct needs: conducting electricity and managing the atmosphere.
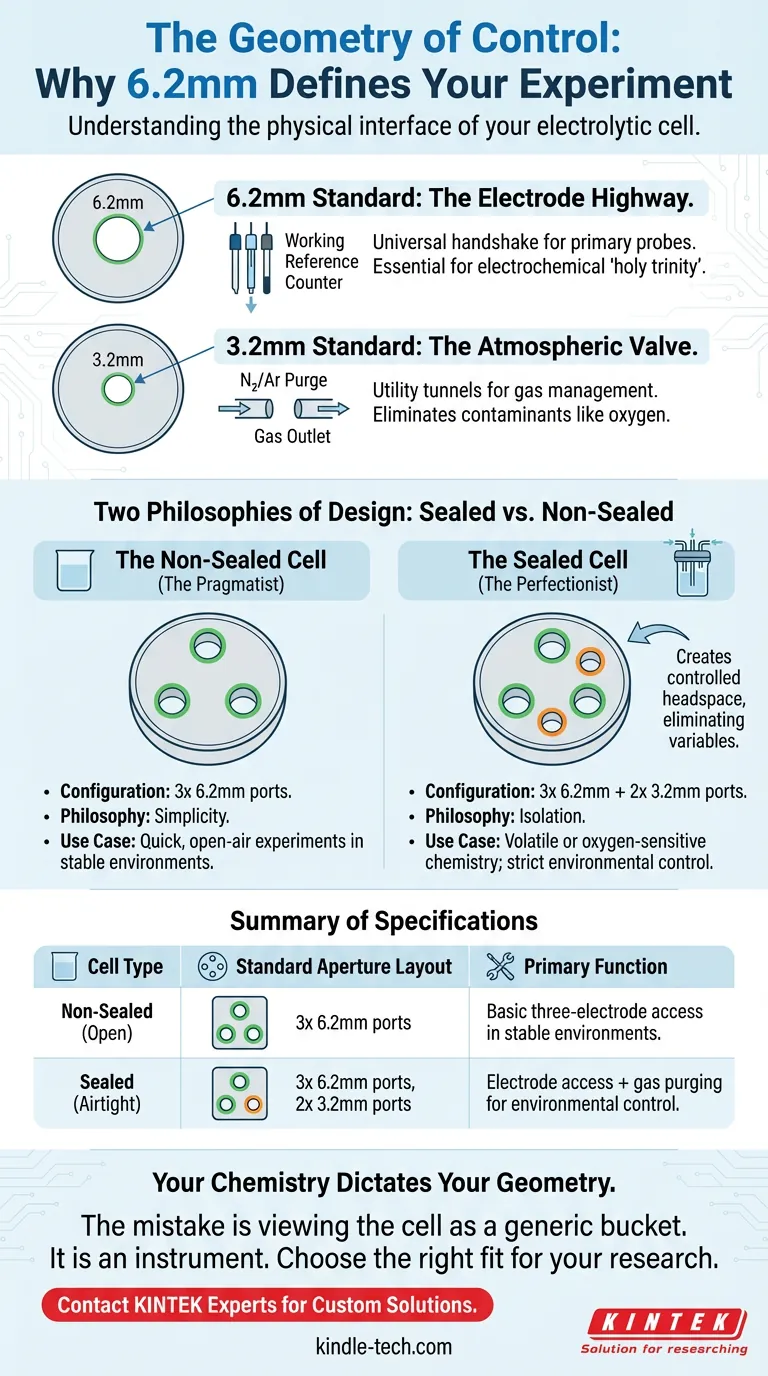
1. The 6.2mm Standard: The Electrode Highway
The most critical number in cell design is 6.2mm.
This is the standard diameter for the primary ports found on almost every electrolytic cell lid. It is the size required to house the "holy trinity" of electrochemistry:
- The Working Electrode
- The Reference Electrode
- The Counter (Auxiliary) Electrode
If you are buying a commercial electrochemical probe, it is likely built to fit a 6.2mm distinct aperture. It is the universal handshake between your potentiostat and your solution.
2. The 3.2mm Standard: The Atmospheric Valve
The second number, 3.2mm, appears when the experiment demands isolation.
These smaller ports are utility tunnels. They are designed for gas inlet and outlet tubes.
In sensitive chemistry, the atmosphere is a contaminant. You need these 3.2mm ports to purge the electrolyte with inert gases like nitrogen or argon, physically pushing out the dissolved oxygen that would otherwise ruin the data.
Two Philosophies of Design
The arrangement of these holes depends entirely on the philosophy of the cell: Sealed vs. Non-Sealed.
This is not just a hardware difference. It represents a choice between simplicity and total control.
The Non-Sealed Cell (The Pragmatist)
For routine aqueous analysis where oxygen interference is negligible, the non-sealed cell is the pragmatist’s choice.
- Configuration: Three 6.2mm ports.
- Philosophy: Simplicity.
- Use Case: Quick, open-air experiments where the environment is not the enemy.
The Sealed Cell (The Perfectionist)
When you move to non-aqueous electrochemistry or study oxygen-sensitive redox couples, the air in the room becomes a variable you must eliminate.
- Configuration: Three 6.2mm ports + Two 3.2mm ports.
- Philosophy: Isolation.
- Use Case: Volatile substances or strict environmental control.
Here, the extra 3.2mm ports allow you to create a controlled headspace, effectively hermetically sealing the experiment from the outside world.
Summary of Specifications
To visualize the trade-offs, we can look at the standard configurations side-by-side:
| Cell Type | Standard Aperture Layout | Primary Function |
|---|---|---|
| Non-Sealed (Open) | 3x 6.2mm ports | Basic three-electrode access in stable environments. |
| Sealed (Airtight) | 3x 6.2mm ports 2x 3.2mm ports |
Electrode access combined with gas purging for environmental control. |
Your Chemistry Dictates Your Geometry
The mistake many researchers make is viewing the cell as a generic bucket.
It is an instrument.
If you choose a non-sealed cell for a sensitive reduction reaction, no amount of data processing will fix the oxygen spike. Conversely, using a complex sealed system for a basic aqueous test introduces unnecessary friction to your workflow.
The Customization Option
It is worth noting that while 6.2mm and 3.2mm are the standards, they are not laws of physics.
Science often happens at the edges of standard protocols. If your research involves unique sensors, oversized probes, or specific sampling requirements, the lid configuration should adapt to you, not the other way around.
The Right Fit for the Job
At KINTEK, we understand that precision engineering is the foundation of reliable chemistry. Whether you need the robust simplicity of a standard open cell or the hermetic security of a sealed system, our equipment is designed to ensure the interface never gets in the way of the innovation.
Do not let a poor fit compromise your data. Contact Our Experts to discuss your specific aperture requirements and find the electrolytic cell that matches your ambition.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
Related Articles
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Fragility of Precision: Mastering the Integrity of Five-Port Electrolytic Cells
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells




















