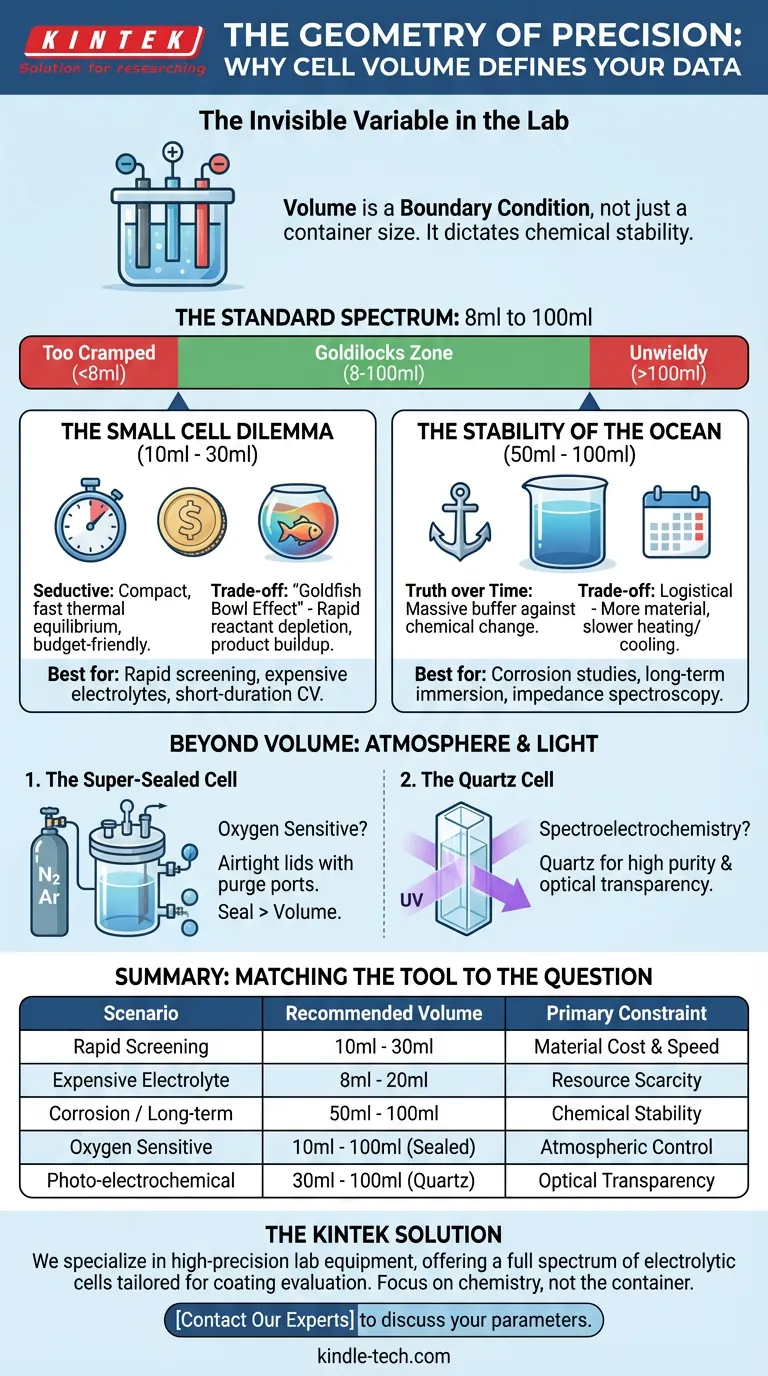
The Invisible Variable in the Lab
In experimental science, we have a tendency to obsess over the protagonist and ignore the stage.
In coating evaluations, the protagonist is your sample—the new alloy, the polymer shield, the corrosion inhibitor. You spend weeks perfecting the synthesis. But the "stage"—the electrolytic cell holding the fluid—is often treated as an afterthought.
This is a mistake.
The volume of your cell is not merely a container size; it is a boundary condition. It dictates the chemical stability of your environment. Whether you choose a standard 8ml vessel or a 100ml bulk reservoir, you are making a specific trade-off between economic efficiency and chemical inertia.
Here is how to navigate that trade-off without compromising your data.
The Standard Spectrum: 8ml to 100ml
For most coating evaluations, the industry standard for electrolytic cells falls between 8ml and 100ml.
This range is not arbitrary. It represents the physical "Goldilocks zone" for the three-electrode system.
- Below 8ml: The geometry becomes too cramped. The working electrode, reference electrode, and counter electrode sit too close together, causing electrical field interference and uneven current distribution.
- Above 100ml: The setup often becomes unwieldy for benchtop screening, requiring excessive amounts of electrolyte for simple pass/fail tests.
Within this range, however, the volume you choose changes the physics of the experiment.
The Small Cell Dilemma (10ml - 30ml)
Small volume cells are seductive. They are compact, they reach thermal equilibrium quickly, and they are kind to your budget.
If you are working with an exotic, expensive electrolyte—or a synthesized fluid you only have in small quantities—a 15ml cell is often the only logical choice.
The Engineering Trade-off: The problem with small volumes is the "Goldfish Bowl Effect." Just as a small fish tank gets dirty quickly, a small volume of electrolyte changes its chemical composition rapidly during a reaction.
- Reactant Depletion: The active species are consumed faster relative to the total volume.
- Product Buildup: Byproducts accumulate quickly, potentially altering the pH or conductivity of the solution mid-test.
Best for: Rapid screening, expensive electrolytes, short-duration cyclic voltammetry.
The Stability of the Ocean (50ml - 100ml)
When you need truth over time, volume is your friend.
Larger cells, typically 50ml to 100ml, provide a massive buffer against chemical change. In a long-term immersion test (like corrosion monitoring over 72 hours), you need the bulk solution to remain chemically constant.
The Engineering Trade-off: The downside is purely logistical. It requires more material. Heating or cooling a 100ml cell takes significantly longer than heating a 20ml cell. It is heavy, slow, and stable.
Best for: Corrosion studies (Tafel plots), long-term immersion, impedance spectroscopy (EIS) where stability is paramount.
Beyond Volume: The Atmosphere and The Light
Once you have determined the volume, you must address the material and the seal. The volume defines the chemistry; the design defines the control.
1. The Super-Sealed Cell
If your coating reacts with oxygen, a standard glass beaker is a liability. Super-sealed cells (typically 10ml – 100ml) feature airtight lids with dedicated ports.
This allows you to purge the headspace with nitrogen or argon. In these scenarios, the seal is more critical than the volume. A 100ml cell that leaks air is useless compared to a 20ml cell that holds a perfect vacuum.
2. The Quartz Cell
Standard glass blocks UV light. If your experiment involves spectroelectrochemistry (analyzing the coating's response to light), you need Quartz.
These are specialized tools, usually available in 30ml to 100ml formats. They offer high purity and optical transparency, allowing you to "see" the chemistry happening on the surface.
Summary: Matching the Tool to the Question
There is no universal "correct" volume. There is only the volume that fits your specific error margins.
Use this decision matrix to simplify your choice:
| Scenario | Recommended Volume | Primary Constraint |
|---|---|---|
| Rapid Screening | 10ml - 30ml | Material Cost & Speed |
| Expensive Electrolyte | 8ml - 20ml | Resource Scarcity |
| Corrosion / Long-term | 50ml - 100ml | Chemical Stability |
| Oxygen Sensitive | 10ml - 100ml (Sealed) | Atmospheric Control |
| Photo-electrochemical | 30ml - 100ml (Quartz) | Optical Transparency |
The KINTEK Solution
At KINTEK, we believe that the equipment should never be the source of experimental error.
We specialize in high-precision lab equipment, offering a full spectrum of electrolytic cells tailored for coating evaluation. Whether you need the economic efficiency of a small-volume sealed cell or the optical clarity of a large quartz vessel, we provide the hardware that allows you to focus on the chemistry, not the container.
Do not let the wrong volume distort your data.
Contact Our Experts to discuss your specific experimental parameters. Let us help you select the precise cell geometry that ensures your results are reproducible, accurate, and true.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Super Sealed Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
Related Articles
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells
- The Architecture of Precision: Mastering Electrolytic Cell Maintenance
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- The Fragility of Precision: Mastering the Integrity of Five-Port Electrolytic Cells




















