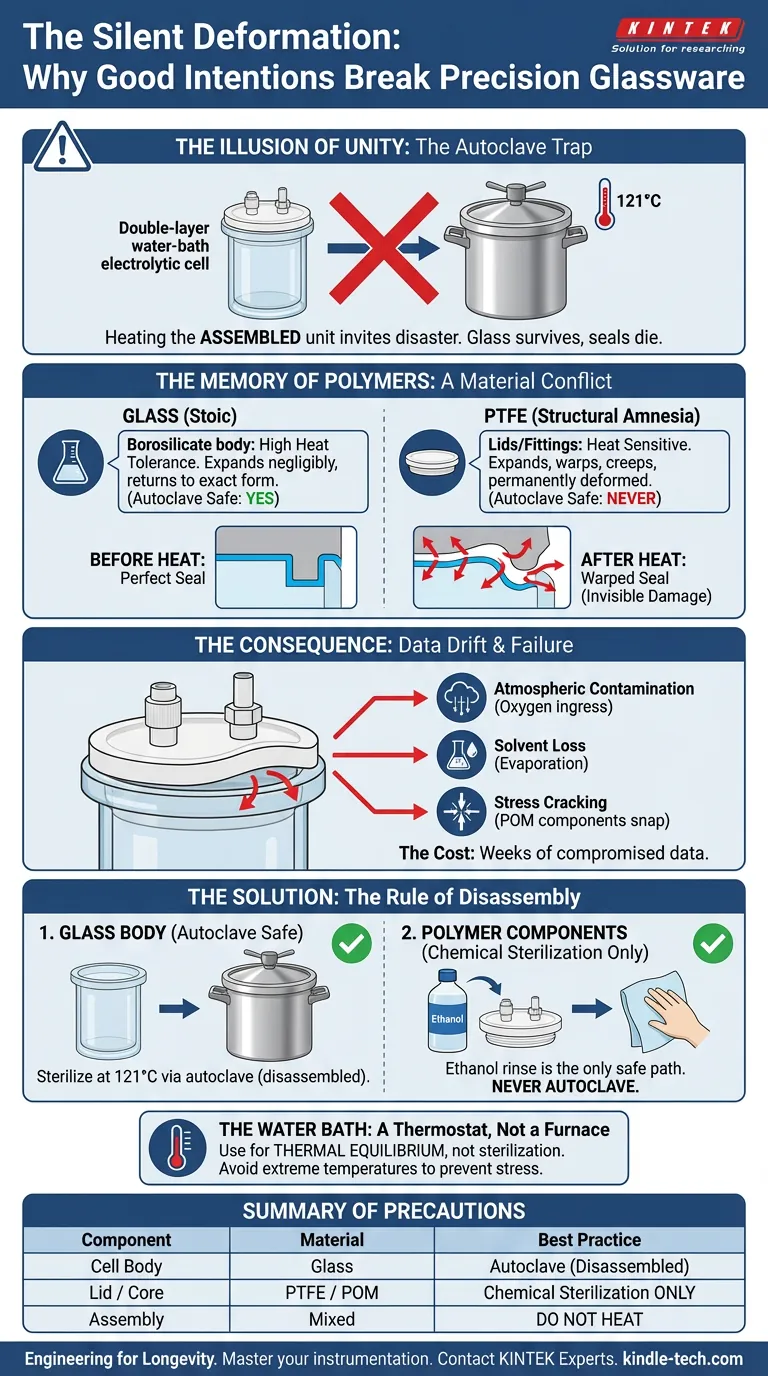
The Illusion of Unity
In the laboratory, we often treat our equipment as monolithic tools. A beaker is a beaker; a cell is a cell. We crave efficiency, and the most efficient way to sterilize equipment is often the most destructive.
The double-layer water-bath electrolytic cell presents a unique engineering paradox. To the eye, it is a single, cohesive unit designed for precision. To the laws of thermodynamics, however, it is a volatile marriage of two opposing materials: silicate glass and fluoropolymers (PTFE).
The most common failure in electrochemical experiments isn't incorrect chemistry. It is the misunderstanding of this relationship.
When we treat the assembly as a single object during sterilization or heating, we invite disaster. The glass survives, but the seals die a silent death.
The Memory of Polymers
The core conflict lies in how materials remember their shape.
Glass is stoic. You can subject the borosilicate body of an electrolytic cell to an autoclave at 121°C, and it remains indifferent. It expands negligibly and returns to its exact form.
PTFE (Polytetrafluoroethylene), used for lids and fittings, is different. It behaves with a kind of structural amnesia.
When you heat a PTFE component significantly—especially while it is constrained within a glass assembly—it expands. But unlike glass, it does not always retreat to its original dimensions. It warps. It creeps.
The Autoclave Trap
This leads to the "Autoclave Trap." A researcher, aiming for perfect sterility, places the assembled cell into the autoclave.
- The Intent: A sterile environment for bio-electrochemical study.
- The Result: A permanently deformed lid.
Once the PTFE lid warps, the geometric tolerance required for a hermetic seal is gone. The damage is often invisible to the naked eye until you run your next experiment and find oxygen leaking into your deoxygenated solution.
The Rule of Disassembly
To navigate this, one must adopt a strict protocol of separation:
- The Glass Body: Can be autoclaved freely at 121°C.
- The Polymer Components: Must never see the inside of an autoclave. Chemical sterilization (such as an ethanol rinse) is the only safe path for the lid and fittings.
The Water Bath: A Thermostat, Not a Furnace
The double-layer design features a jacketed glass body intended for connection to a water bath. This is often mistaken for a heating element.
It is not. It is a control mechanism.
The purpose of the water bath is to maintain a thermal equilibrium—keeping the electrolyte temperature strictly consistent to ensure reaction kinetics are governed by chemistry, not temperature fluctuations.
Managing Thermal Stress
Pushing the water bath to extreme temperatures introduces two risks:
- Differential Expansion: The glass jacket and the inner reaction chamber may experience stress if temperatures change too rapidly or become too extreme.
- Safety Hazards: The apparatus is not insulated. A 90°C water bath turns the cell into a burn hazard, requiring strict PPE protocols.
The Cost of "Good Enough"
Why does this matter? Because in electrochemistry, a leak is not just a leak. It is data drift.
A deformed PTFE lid leads to:
- Atmospheric Contamination: Oxygen ingress alters reduction potentials.
- Solvent Loss: Evaporation changes electrolyte concentration over time.
- Stress Cracking: POM (Polyoxymethylene) components may simply snap under thermal load.
The cost isn't just the replacement price of the lid. It is the weeks of data generated by a compromised instrument that must now be discarded.
Summary of Precautions
To maintain the longevity of your KINTEK electrolytic cell, treat the components according to their nature, not their proximity.
| Component | Material | Thermal Capability | Best Practice |
|---|---|---|---|
| Cell Body | Glass | High (Autoclave Safe) | Sterilize at 121°C via autoclave (disassembled). |
| Lid / Core | PTFE / POM | Low (Heat Sensitive) | Chemical sterilization only. Never autoclave. |
| Assembly | Mixed | Do Not Heat | Never heat the fully assembled unit. |
| Jacket | Glass | Moderate Control | Use for stabilizing reaction temp, not sterilization. |
Engineering for Longevity
Precision requires respect for materials. The double-layer electrolytic cell is a sophisticated tool designed for specific thermal environments. By understanding the distinct limits of glass and polymer, you move from simply running experiments to mastering your instrumentation.
At KINTEK, we design our equipment to withstand the rigors of serious science, but we rely on the researcher's hand to maintain them. Whether you need durable glass bodies or replacement polymer fittings, our inventory is built to support your lab's precision.
Do not let invisible deformation compromise your results. Contact Our Experts today to discuss the right maintenance protocols or to upgrade your electrochemical setup.
Visual Guide
Related Products
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Super Sealed Electrolytic Electrochemical Cell
Related Articles
- The Architecture of Precision: Mastering the Five-Port Water Bath Electrolytic Cell
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques
- Exploring the Multifunctional Electrolytic Cell Water Bath: Applications and Benefits