The most dangerous moment in a laboratory is rarely when an alarm is blaring. It is when everything looks quiet, but the physics of the system are slowly drifting beyond the point of no return.
In electrochemistry, we often conflate intensity with efficiency.
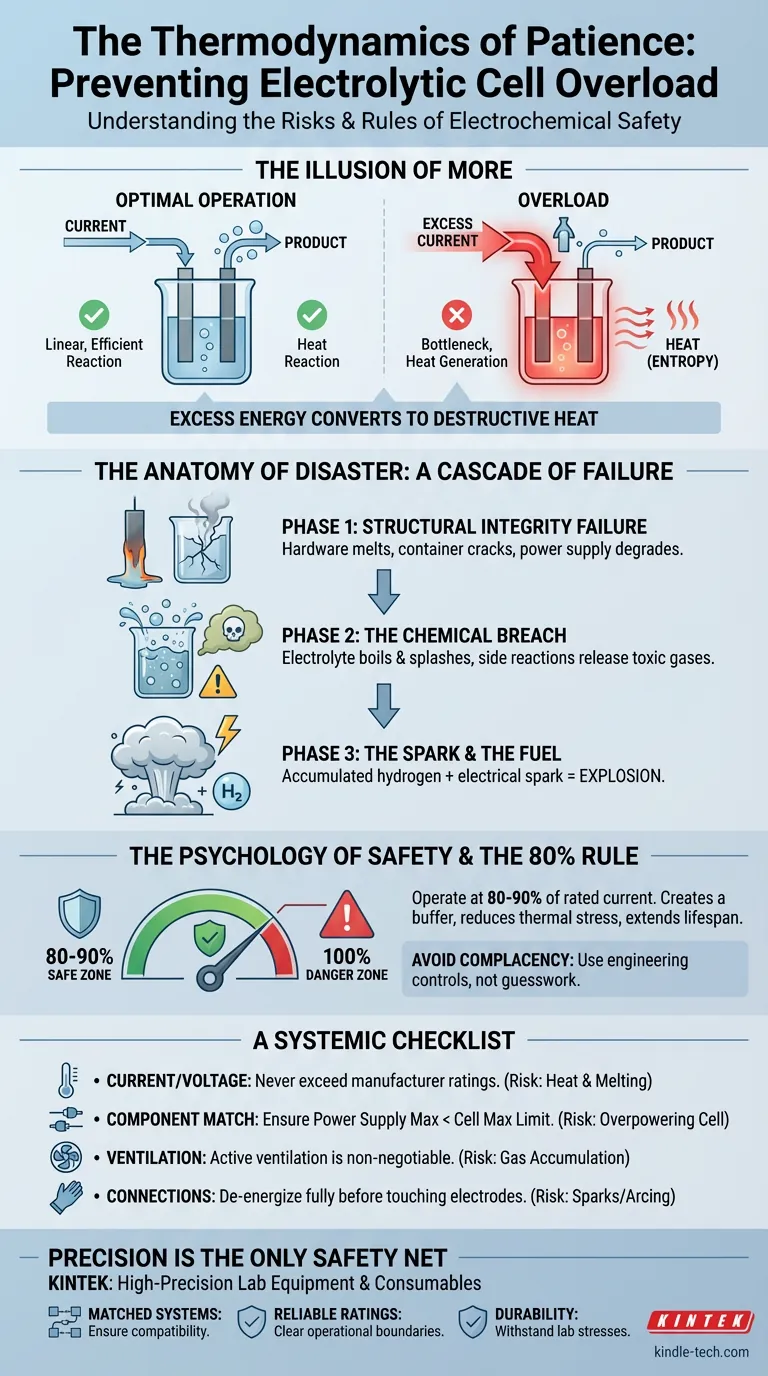
We assume that if a specific current yields a reaction, more current will yield the reaction faster. But electrolytic cells do not operate on linear optimism. They operate on strict thermodynamic thresholds.
When you push an electrolytic cell beyond its rated capacity, you aren't just speeding up a process. You are changing the nature of the energy transfer. You are converting useful work into destructive entropy.
The Illusion of More
An electrolytic cell and its power supply must be viewed as a single, integrated biological system.
The "Overload" is not merely a dial turned too far to the right. It is a fundamental mismatch between the energy supplied and the system's ability to metabolize it.
When you exceed the rated voltage or amperage:
- The reaction creates a bottleneck. The chemical process hits a speed limit.
- Energy refuses to disappear. Thermodynamics dictates that energy cannot be destroyed.
- Transformation occurs. The excess energy transforms immediately into heat.
This is the engineer's nightmare: You are no longer running an electrolysis experiment; you are effectively running a heater inside a chemical bath.
The Anatomy of Disaster
The risks of overloading are often categorized as "equipment damage," but that phrase is too clinical. It hides the violence of the failure modes.
When the system overheats, the failure cascades through three distinct physical phases:
Phase 1: Structural Integrity Failure
The first victim is the hardware. Intense heat melts electrodes and cracks the cell container. The power supply, straining to deliver current into a chaotic load, begins to degrade.
Phase 2: The Chemical Breach
As the electrolyte boils, it becomes a projectile hazard. Boiling acids or bases do not stay in the beaker; they splash. Furthermore, when pushed beyond intended parameters, the chemistry changes. You may trigger side reactions that release toxic gases distinct from your intended product.
Phase 3: The Spark and The Fuel
This is the catastrophic endpoint. Most electrolytic processes involve the generation of hydrogen.
- The Fuel: Accumulated hydrogen gas.
- The Ignition: An overloaded circuit creates a short or a spark.
The result is not a fire. It is an explosion.
The Psychology of Safety
Why do we overload systems?
Usually, it comes down to complacency. We use a power supply rated for 10 Amps on a cell rated for 2 Amps because "it’s what was on the bench." We assume we can control the dial.
But safety requires engineering controls, not just good intentions.
The 80% Rule
In engineering, reliability is found in the margins.
If you operate equipment at 100% of its rating, you are red-lining the engine. The slightest fluctuation causes failure.
The Golden Rule: Operate your system at 80-90% of its maximum rated current.
This buffer zone reduces thermal stress. It extends the lifespan of your electrodes. Most importantly, it gives you room for error.
A Systemic Checklist
Safe electrolysis is not about luck; it is about rigid adherence to variables.
| Variable | The Risk | The Protocol |
|---|---|---|
| Current/Voltage | Heat generation & Melting | Never exceed manufacturer ratings. |
| Component Match | Overpowering the cell | Ensure Power Supply Max < Cell Max Limit. |
| Ventilation | Gas accumulation | Active ventilation is non-negotiable. |
| Connections | Sparks/Arcing | De-energize fully before touching electrodes. |
Precision is the Only Safety Net
The difference between a successful synthesis and a lab accident is often a matter of millivolts and degrees.
You cannot manage these invisible forces with guesswork. You need equipment that offers precise regulation and robust safety margins.
This is where KINTEK steps in.
We understand that an electrolytic cell is only as safe as the ecosystem it inhabits. KINTEK specializes in high-precision lab equipment and consumables designed to handle the rigors of electrochemical work.
- Matched Systems: We help you pair power supplies with cells to ensure compatibility.
- Reliable Ratings: Our specifications are rigorous, giving you clear boundaries for safe operation.
- Durability: Materials designed to withstand the thermal and chemical stresses of the lab.
Do not leave your safety to chance or mismatched components.
Contact Our Experts to audit your setup and find the precise KINTEK equipment that keeps your lab safe, efficient, and firmly within the limits of physics.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Super Sealed Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
Related Articles
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Symphony of Coefficients: Why Your Electrolytic Cell Cannot Be a Monolith
- The Fragility of Precision: Mastering the Integrity of Five-Port Electrolytic Cells




















