The most dangerous moment in any electrochemical experiment is not when the reaction fails. It is when the reaction succeeds, but the data is wrong.
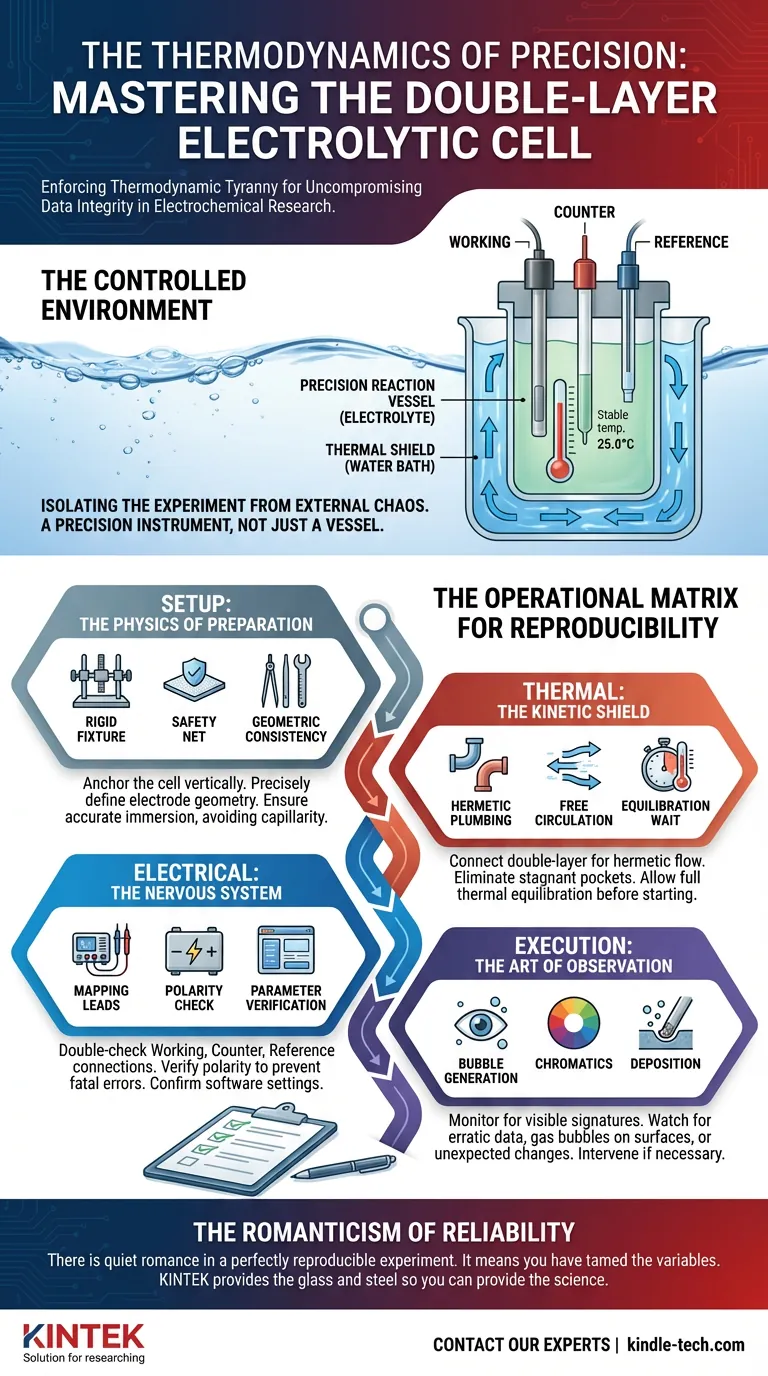
We often view the electrolytic cell as a simple vessel—a bucket for chemistry. This is a mistake. The double-layer water-bath electrolytic cell is a precision instrument designed to isolate your experiment from the chaos of the outside world.
Its primary function is not just to hold liquid, but to enforce thermodynamic tyranny. It dictates the temperature, ensuring that the only variable changing is the one you are testing.
Operating it requires a shift in mindset. You are not just mixing chemicals; you are constructing a controlled environment. As Atul Gawande might suggest, the difference between a breakthrough and background noise is often a checklist.
The Physics of Preparation
Most experiments fail before the power supply is ever turned on. They fail in the physical setup.
Stability is the prerequisite for accuracy. A wobbling cell introduces variables you cannot account for.
- The Physical Anchor: Secure the cell to the laboratory stand. Tighten the knobs until the unit is vertically rigid.
- The Safety Net: If you are working with corrosive electrolytes, placing a leak-proof pad underneath is not paranoia—it is insurance.
- The Geometry of Reaction: Install the working, counter, and reference electrodes. Their spacing defines the electric field. If the spacing changes between experiments, your data is no longer comparable.
Note: Electrode immersion is a balancing act. The active surface must be submerged, but the conductive rods must remain dry. Capillarity is a subtle enemy; ensure the electrolyte level is precise.
The Thermal Shield: Configuring the Water Bath
The defining feature of this equipment is the "double layer"—the jacket. This is your thermal shield.
Chemistry is slave to temperature. A fluctuation of a few degrees can alter reaction kinetics, diffusivity, and viscosity. The water bath eliminates this variable.
- Plumbing: Connect the inlet and outlet pipes. These connections must be hermetic. A water leak is not just a mess; it is a breach of thermal integrity.
- Circulation: Ensure the water flows freely. Stagnant pockets of water create thermal gradients.
- The Wait: Set the temperature and wait. This is the hardest part for the eager engineer. The electrolyte inside the cell needs time to reach thermal equilibrium with the jacket.
The Electrical Nervous System
The electrochemical workstation is the brain; the wires are the nerves. If the nerves are crossed, the system collapses.
Connecting the electrode leads requires deliberate attention.
- Mapping: Verify the Working, Counter, and Reference leads.
- Polarity: Check the main power supply. Reversing polarity is not a minor error; it is often a fatal blow to sensitive electrodes or the cell itself.
Once the physical and electrical systems are linked, double-check your software parameters. Scanning voltage, potential windows, and current ranges must be typed in, read, and read again.
The Art of Observation
Once you hit "Start," your role shifts from architect to observer.
The software will capture the quantitative data—current, potential, time. But it cannot see what is happening. You must provide the qualitative context.
Watch for the visible signatures of chemistry:
- Bubble generation: Is gas evolving at the expected rate?
- Chromatics: Is the solution changing color?
- Deposition: Is matter forming on the electrode surface?
If the system behaves erratically—unexpected noise in the data or sudden spikes—intervention is necessary. A loose connection or a gas bubble trapped on an electrode face can ruin an hour of work.
Operational Matrix
Success in electrochemistry is rarely about brilliance. It is about removing sources of failure.
| Phase | The "Silent" Risk | The Engineering Solution |
|---|---|---|
| Setup | Geometric inconsistency | Rigidly fix electrode spacing and depth. |
| Thermal | Kinetic fluctuation | Allow full equilibration time before starting. |
| Electrical | Reversed polarity | Double-verify lead connections to the workstation. |
| Execution | Unnoticed anomalies | Visually monitor electrode surfaces for bubbles/deposits. |
Conclusion: The Romanticism of Reliability
There is a quiet romance in a perfectly reproducible experiment. It means you have tamed the variables.
The double-layer electrolytic cell is the tool that allows you to assert this control. But the tool is only as good as the hands that set it up.
At KINTEK, we understand that your research is built on the integrity of your equipment. We provide the glass and steel so you can provide the science.
Contact Our Experts to discuss how our precision electrochemical equipment can stabilize your research and secure your data.
Visual Guide
Related Products
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Super Sealed Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
Related Articles
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Architecture of Control: Why Thermal Stability Defines Electrolysis Success
- The Architecture of Precision: Mastering the Five-Port Water Bath Electrolytic Cell
- The Glass Heart of the Experiment: Precision Through Systematic Care
- Exploring the Multifunctional Electrolytic Cell Water Bath: Applications and Benefits




















