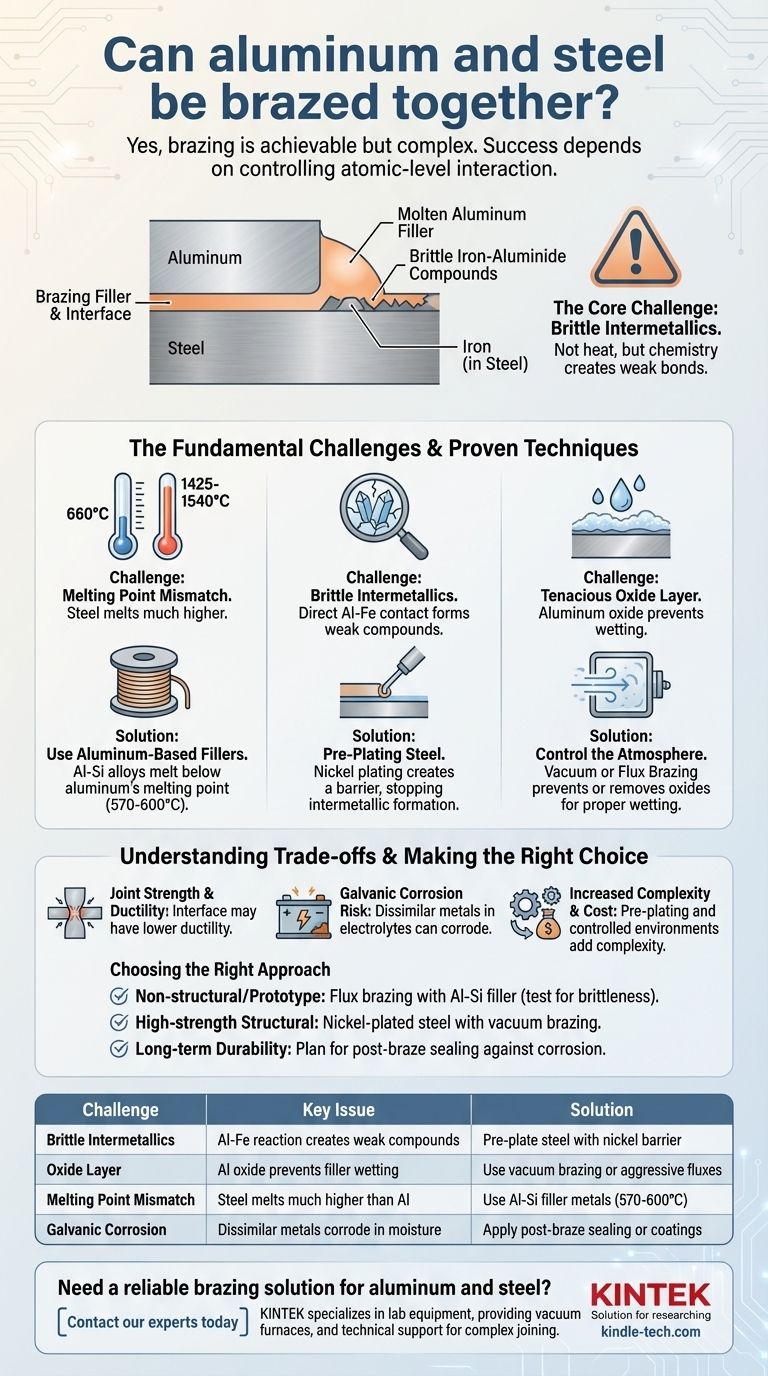
Yes, brazing aluminum to steel is achievable, but it is a complex process that demands specific techniques. Unlike brazing similar metals, joining aluminum and steel requires careful management of their fundamentally different properties to prevent a weak, brittle bond. Success hinges on controlling the interaction between the two materials at the atomic level.
The core challenge in brazing aluminum to steel is not the heat, but the chemistry. Direct contact between molten aluminum filler and steel creates brittle iron-aluminide compounds. The solution lies in using specialized aluminum-based fillers and creating a barrier, often by pre-plating the steel, to prevent this destructive reaction.
The Fundamental Challenge: Why This Joint is Difficult
Successfully joining these two metals requires a deep understanding of why they naturally resist forming a strong bond. Three primary factors are at play.
The Melting Point Mismatch
Steel melts at a much higher temperature (around 1425-1540°C or 2600-2800°F) than aluminum (around 660°C or 1220°F).
This means the brazing process must occur below aluminum's melting point. Consequently, you must use a filler metal, typically an aluminum-silicon alloy, that melts at an even lower temperature.
The Problem of Brittle Intermetallics
This is the most critical metallurgical hurdle. When molten aluminum comes into direct contact with iron (the primary component of steel), they react to form iron-aluminide intermetallic compounds.
These compounds are extremely hard and brittle. A thick intermetallic layer in the joint acts as a crack initiation site, leading to catastrophic failure under even minor stress or vibration.
The Tenacious Oxide Layer
Aluminum instantly forms a tough, transparent layer of aluminum oxide (Al₂O₃) on its surface. This oxide has a very high melting point and prevents the braze filler from "wetting" or bonding to the underlying metal.
Steel also oxidizes, but the aluminum oxide layer is particularly stubborn. Overcoming it requires either aggressive chemical fluxes or a controlled atmosphere, such as a vacuum, to prevent its formation in the first place.
Proven Techniques for a Successful Bond
Engineers have developed several effective strategies to overcome the challenges of joining aluminum and steel. These methods focus on controlling the joint's chemistry and environment.
Using Aluminum-Based Fillers
The choice of filler metal is non-negotiable. You must use a brazing alloy with a melting point lower than that of aluminum.
The most common choice is an aluminum-silicon (Al-Si) filler metal. These alloys are designed to flow at temperatures between 570-600°C, safely below the point where the aluminum base metal would melt.
Pre-Plating the Steel Surface
The most reliable method for preventing brittle intermetallics is to create a barrier. This is often done by pre-plating the steel component with a more compatible metal, such as nickel.
The aluminum filler then bonds to the nickel plating instead of the iron. The nickel layer acts as a diffusion barrier, physically separating the aluminum and iron and stopping the formation of brittle compounds.
Controlling the Brazing Atmosphere
To combat the persistent aluminum oxide layer, the brazing must be done in a controlled environment.
Vacuum brazing is highly effective, as removing oxygen prevents oxides from forming on either metal. Alternatively, flux brazing uses aggressive chemical fluxes to dissolve the oxide layer, allowing the filler metal to properly wet and flow into the joint. Automatic brazing machines are often designed to manage these controlled environments precisely.
Understanding the Trade-offs and Limitations
While a strong joint is possible, it's crucial to be aware of the potential compromises and risks associated with any aluminum-to-steel bond.
Joint Strength and Ductility
Even with perfect execution, a brazed aluminum-to-steel joint may not possess the same ductility as a joint between similar metals. The interface between dissimilar materials will always be a potential point of stress concentration.
Galvanic Corrosion Risk
When two different metals like aluminum and steel are in electrical contact in the presence of an electrolyte (like humidity or rainwater), a galvanic cell is created.
This leads to galvanic corrosion, where the more active metal (aluminum) corrodes at an accelerated rate. Over time, this can degrade the integrity of the joint, especially in harsh environments.
Increased Complexity and Cost
The need for pre-plating, specialized filler metals, and controlled atmosphere furnaces makes brazing aluminum to steel significantly more complex and costly than conventional steel-to-steel brazing.
Making the Right Choice for Your Application
Selecting the correct approach depends entirely on the demands of your specific project.
- If your primary focus is a non-structural join or prototype: A carefully executed flux brazing process with an Al-Si filler may be sufficient, but you must rigorously test the joint for brittleness.
- If your primary focus is high-strength structural performance: Using a nickel-plated steel component combined with vacuum brazing is the most robust and reliable method for ensuring joint integrity.
- If your primary focus is long-term durability in a corrosive environment: You must plan for post-braze sealing or coating to protect the joint from moisture and mitigate the inevitable risk of galvanic corrosion.
By understanding the unique metallurgical challenges and deliberately selecting the right process, you can successfully create a reliable bond between aluminum and steel.
Summary Table:
| Challenge | Key Issue | Solution |
|---|---|---|
| Brittle Intermetallics | Molten aluminum reacts with iron to form weak compounds | Pre-plate steel with nickel to create a barrier |
| Oxide Layer | Aluminum's tough oxide prevents filler wetting | Use vacuum brazing or aggressive fluxes |
| Melting Point Mismatch | Steel melts at much higher temps than aluminum | Use Al-Si filler metals (570-600°C melting point) |
| Galvanic Corrosion | Dissimilar metals corrode when exposed to moisture | Apply post-braze sealing or protective coatings |
Need a reliable brazing solution for aluminum and steel?
Brazing dissimilar metals requires precision equipment and expert knowledge. KINTEK specializes in lab equipment and consumables, providing the vacuum furnaces, controlled atmosphere systems, and technical support needed for complex joining processes. Our solutions help you achieve strong, durable bonds while minimizing the risk of brittle intermetallics and corrosion.
Contact our experts today to discuss how we can optimize your brazing process for aluminum-steel applications!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What role does a vacuum diffusion bonding furnace play? Master High-Performance Titanium Laminate Fabrication
- What features must a vacuum furnace have for Cr2AlC MAX phase coatings? Precision Controls for High-Purity Synthesis
- What is the voltage of a vacuum arc? Discover the Low, Stable Voltage for Superior Performance
- Why is a high vacuum furnace required for annealing ferritic alloys at 1100°C? Ensure Purity and Data Integrity
- What is the role of a laboratory vacuum arc remelting furnace? Mastering High-Entropy Alloy Synthesis
- How hot can a lab furnace get? Match the Right Heating Technology to Your Application
- Can you anneal multiple times? Mastering the Cycle for Perfect Metalwork
- What is the function of a precision isothermal heating furnace in inducing secondary phase precipitation? Optimize Microstructures



















