Yes, gold absolutely can turn into vapor. Like virtually all elements, gold will transition from a solid to a liquid and finally to a gas if subjected to enough heat. However, the temperatures required are incredibly high, which is why we never witness this phenomenon in everyday life.
Gold's ability to become a vapor is a fundamental property of matter, but its extremely high boiling point is precisely what makes it so remarkably stable and valuable as a material on Earth.

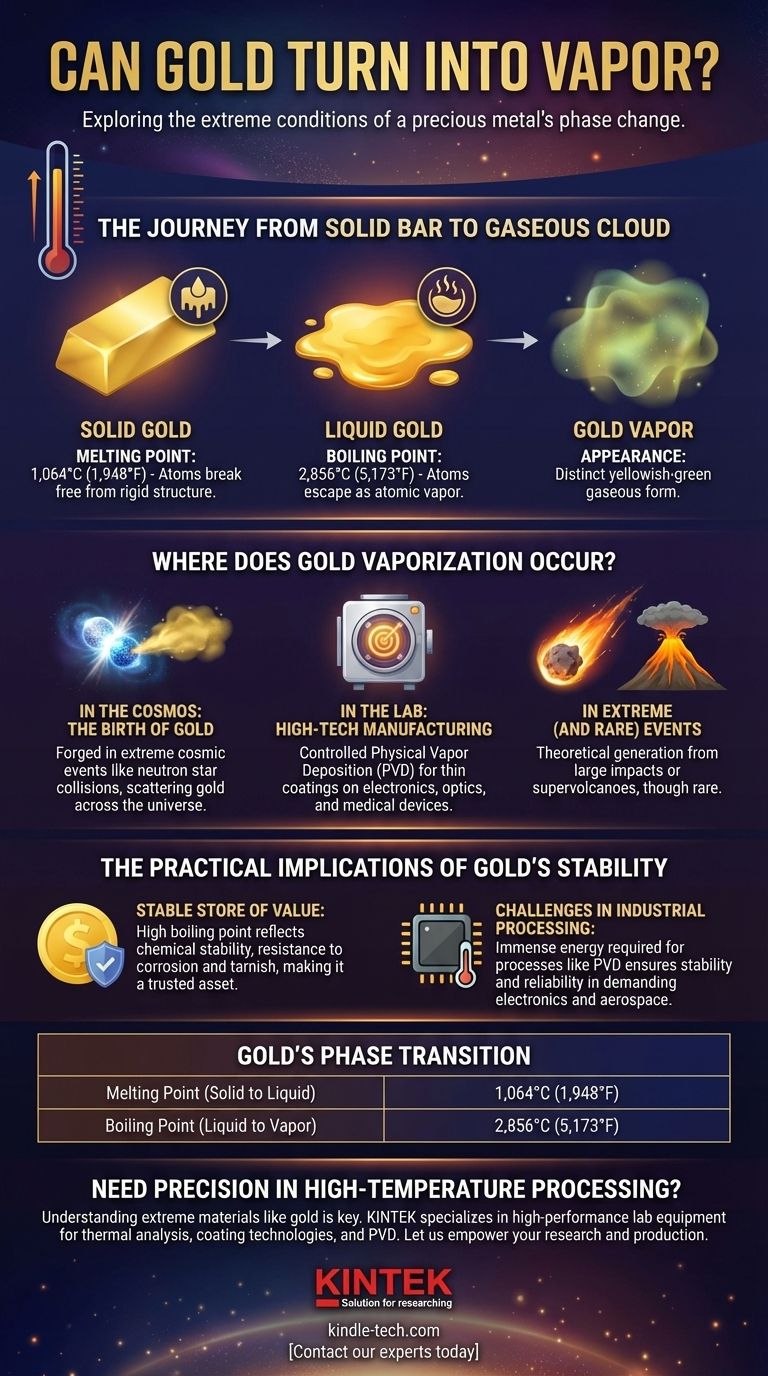
From Solid Bar to Gaseous Cloud: The Journey of Gold
To understand how gold vaporizes, we must look at its specific transition points. These temperatures are a measure of the energy needed to break the strong metallic bonds holding its atoms together.
The Melting Point: Becoming a Liquid
First, for gold to even begin the journey to becoming a vapor, it must melt into a liquid.
This occurs at 1,064°C (1,948°F). At this temperature, the atoms have enough energy to break free from their rigid crystal structure but still remain loosely bonded.
The Boiling Point: Becoming a Vapor
To turn liquid gold into a gas, the temperature must be raised significantly higher.
Gold's boiling point is approximately 2,856°C (5,173°F). For context, this is hotter than the surface of many small stars. At this point, the atoms gain enough energy to sever all bonds and escape as an atomic vapor.
The Appearance of Gold Vapor
When gold does vaporize, it doesn't appear as a shimmering golden cloud.
Scientists observe that gaseous gold often has a distinct yellowish-green or greenish color, a fascinating contrast to its familiar metallic luster in solid form.
Where Does Gold Vaporization Actually Occur?
Since these temperatures are so extreme, the vaporization of gold is confined to very specific and powerful environments. It doesn't happen naturally on Earth's surface.
In the Cosmos: The Birth of Gold
The most dramatic example of gold vaporization happens in space.
Gold itself is forged in catastrophic cosmic events like the collision of neutron stars. The immense energy of these events creates and vaporizes gold and other heavy elements, which are then scattered across the cosmos.
In the Lab: High-Tech Manufacturing
On Earth, gold is vaporized under controlled conditions for advanced industrial applications.
Processes like physical vapor deposition (PVD) heat gold in a vacuum until it turns into a gas. This vapor is then used to apply incredibly thin, uniform coatings of gold onto surfaces, a critical step in making electronics, specialized optics, and medical devices.
In Extreme (and Rare) Events
Theoretically, a sufficiently powerful event like a large meteorite impact or a supervolcano could generate the temperatures needed to vaporize gold-bearing rock.
However, these are not common occurrences and remain largely in the realm of geological theory.
The Practical Implications of Gold's Stability
The immense difficulty in vaporizing gold is not a limitation; it is one of its most important features. This resistance to change is directly linked to its value and utility.
Why Gold is a Stable Store of Value
Gold's high melting and boiling points are a physical manifestation of its chemical stability.
It doesn't easily corrode, tarnish, or react with other elements. This permanence and inability to be easily destroyed or altered is a primary reason it has been a trusted store of value for millennia.
Challenges in Industrial Processing
The energy required to work with gold at these temperatures is immense.
While this makes industrial processes like PVD complex and expensive, it also ensures that gold components in electronics or aerospace applications remain stable and reliable even under demanding conditions.
How This Applies to Your Understanding
Your interest in gold's properties can be viewed from several angles, each with a key takeaway.
- If your primary focus is chemistry and physics: Remember that gold's state is a function of energy, and its high boiling point reflects the strength of its metallic bonds.
- If your primary focus is its value and history: Recognize that gold's physical durability and resistance to vaporization are the scientific basis for its economic role as a stable asset.
- If your primary focus is technology: Understand that vaporizing gold is a key industrial process used to create the ultra-thin, precise coatings essential for modern electronics.
Ultimately, gold's ability to become a vapor underscores the extreme conditions under which it was born and the remarkable stability that makes it so precious on Earth.
Summary Table:
| Gold's Phase Transition | Temperature |
|---|---|
| Melting Point (Solid to Liquid) | 1,064°C (1,948°F) |
| Boiling Point (Liquid to Vapor) | 2,856°C (5,173°F) |
Need Precision in High-Temperature Processing?
Understanding the extreme properties of materials like gold is at the heart of advanced manufacturing. At KINTEK, we specialize in the high-performance lab equipment and consumables needed to explore and manipulate materials under demanding conditions—from thermal analysis to coating technologies.
Whether you're developing next-generation electronics, specialized optics, or medical devices, our solutions support the precise control required for processes like Physical Vapor Deposition (PVD).
Let KINTEK empower your research and production. Contact our experts today to discuss how our reliable equipment can meet your laboratory's specific challenges.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- Can I vacuum the inside of my furnace? A Guide to Safe DIY Cleaning vs. Professional Service
- What is the vacuum heat treatment cycle? Achieve Superior Material Purity and Precision
- What is a vacuum furnace used for? Unlock High-Purity Heat Treatment for Superior Materials
- What is the structure of a vacuum furnace? A Guide to Its Core Components & Functions
- What is the leak rate for a vacuum furnace? Ensure Process Purity and Repeatability



















