The most direct way to avoid contamination is to prevent external impurities, such as dust and other particles, from entering the electrolytic cell. This is achieved by maintaining a clean experimental area and avoiding any operations nearby that could generate airborne contaminants, thereby ensuring the purity of the electrolyte is maintained throughout the experiment.
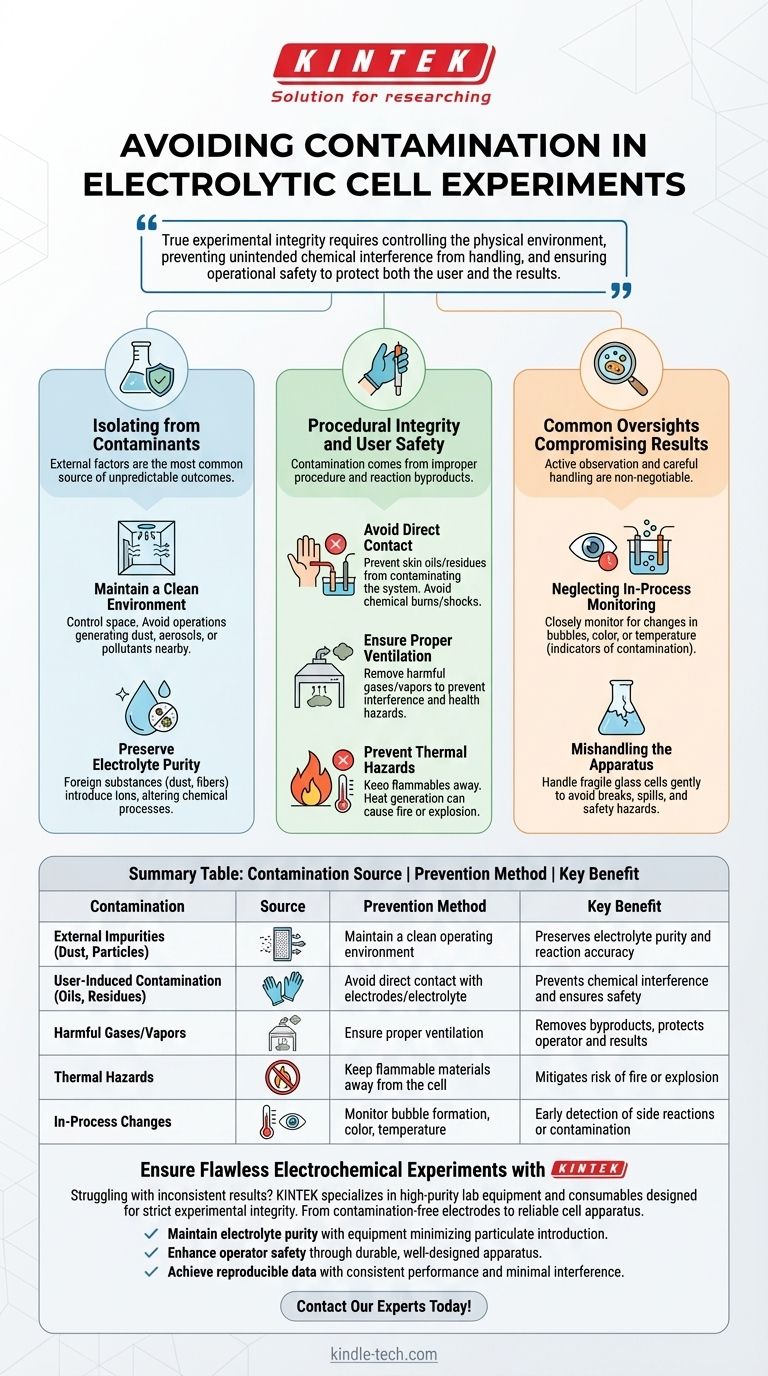
The core challenge is recognizing that "contamination" extends beyond simple dust. True experimental integrity requires controlling the physical environment, preventing unintended chemical interference from handling, and ensuring operational safety to protect both the user and the results.

Isolating the Experiment from Contaminants
The integrity of any electrochemical experiment hinges on the purity of its components. External factors are the most common source of unpredictable and undesirable outcomes.
Maintain a Clean Operating Environment
The most fundamental step is to control the space around the cell. Avoid conducting any operations that generate dust, aerosols, or other pollutants in the immediate vicinity of your setup.
Preserve Electrolyte Purity
The electrolyte is the heart of the cell. Any foreign substance, from a spec of dust to a microscopic fiber, can introduce new ions or reaction sites, fundamentally altering the intended chemical process and compromising the accuracy of your results.
Procedural Integrity and User Safety
Contamination isn't just about what falls into your cell; it's also about what you introduce through improper procedure or what the reaction itself produces. These factors can skew results and create significant hazards.
Avoid Direct Physical Contact
You must avoid touching the electrodes or the electrolyte directly. Not only does this prevent potential chemical burns or electric shock, but it also prevents oils, salts, and other residues from your skin from contaminating the highly sensitive chemical system.
Ensure Proper Ventilation
Electrolysis can produce harmful gases or vapors as byproducts. Proper ventilation is critical to remove these substances, preventing them from interfering with the experiment or posing a poisoning hazard to the operator.
Prevent Thermal Hazards
Electrolytic processes can generate heat. Keep flammable materials and open flames far away from the cell to mitigate the risk of fire or explosion, which represents the ultimate form of experimental contamination and failure.
Common Oversights That Compromise Results
Even with a clean environment, inattention during the experiment can lead to failure. Active observation and careful handling are non-negotiable for achieving reliable data.
Neglecting In-Process Monitoring
You must closely monitor the working state of the cell throughout the experiment. Watch for changes in bubble formation on the electrodes, shifts in electrolyte color, or fluctuations in temperature. These are often the first indicators of an unexpected side reaction or contamination.
Mishandling the Apparatus
The cell body is often made of fragile materials like glass. It must be handled gently at all times. A crack or break not only ruins the experiment but also creates a significant safety hazard from spills and broken glass.
A Checklist for Maintaining Experimental Integrity
Your approach should be tailored to the primary goal of your work, whether it's maximizing precision, ensuring safety, or achieving repeatable results.
- If your primary focus is analytical accuracy: Prioritize a meticulously clean environment and prevent any foreign particles from entering the cell to maintain electrolyte purity.
- If your primary focus is operator safety: Emphasize proper ventilation and strict adherence to no-contact protocols for handling the electrodes and electrolyte.
- If your primary focus is reproducibility: Implement a rigorous monitoring protocol to observe and log all changes within the cell during operation.
A disciplined and systematic approach is the only way to guarantee the integrity of your experimental results.
Summary Table:
| Contamination Source | Prevention Method | Key Benefit |
|---|---|---|
| External Impurities (Dust, Particles) | Maintain a clean operating environment | Preserves electrolyte purity and reaction accuracy |
| User-Induced Contamination (Oils, Residues) | Avoid direct contact with electrodes/electrolyte | Prevents chemical interference and ensures safety |
| Harmful Gases/Vapors | Ensure proper ventilation | Removes byproducts, protects operator and results |
| Thermal Hazards | Keep flammable materials away from the cell | Mitigates risk of fire or explosion |
| In-Process Changes | Monitor bubble formation, color, temperature | Early detection of side reactions or contamination |
Ensure Flawless Electrochemical Experiments with KINTEK
Struggling with inconsistent results or contamination in your electrolytic cell experiments? KINTEK specializes in high-purity lab equipment and consumables designed to uphold the strictest standards of experimental integrity. From contamination-free electrodes to reliable cell apparatus, our products help you:
- Maintain electrolyte purity with equipment that minimizes particulate introduction.
- Enhance operator safety through durable, well-designed apparatus that reduces handling risks.
- Achieve reproducible data with consistent performance and minimal interference.
Let us help you eliminate variables and focus on your research. Contact our experts today to find the right solutions for your laboratory's unique needs!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the standard aperture sizes on the lid of the multifunctional electrolytic cell? Key Ports for Your Electrochemical Setup
- What are the key features of the five-port water bath electrolytic cell? Precision Control for Electrochemical Experiments
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What are the procedures for after using a double-layer water-bath electrolytic cell? Ensure Equipment Longevity and Data Accuracy
- What are the standard aperture specifications of the electrolytic cell? Key Sizes for Your Electrochemical Setup



















