In short, these black or brown substances are typically removed by chemical cleaning. The standard procedure involves soaking the platinum mesh electrode in a dilute acid, such as nitric or hydrochloric acid. For more stubborn contaminants, this chemical treatment can be combined with ultrasonic cleaning to physically dislodge the deposits from the electrode's surface.
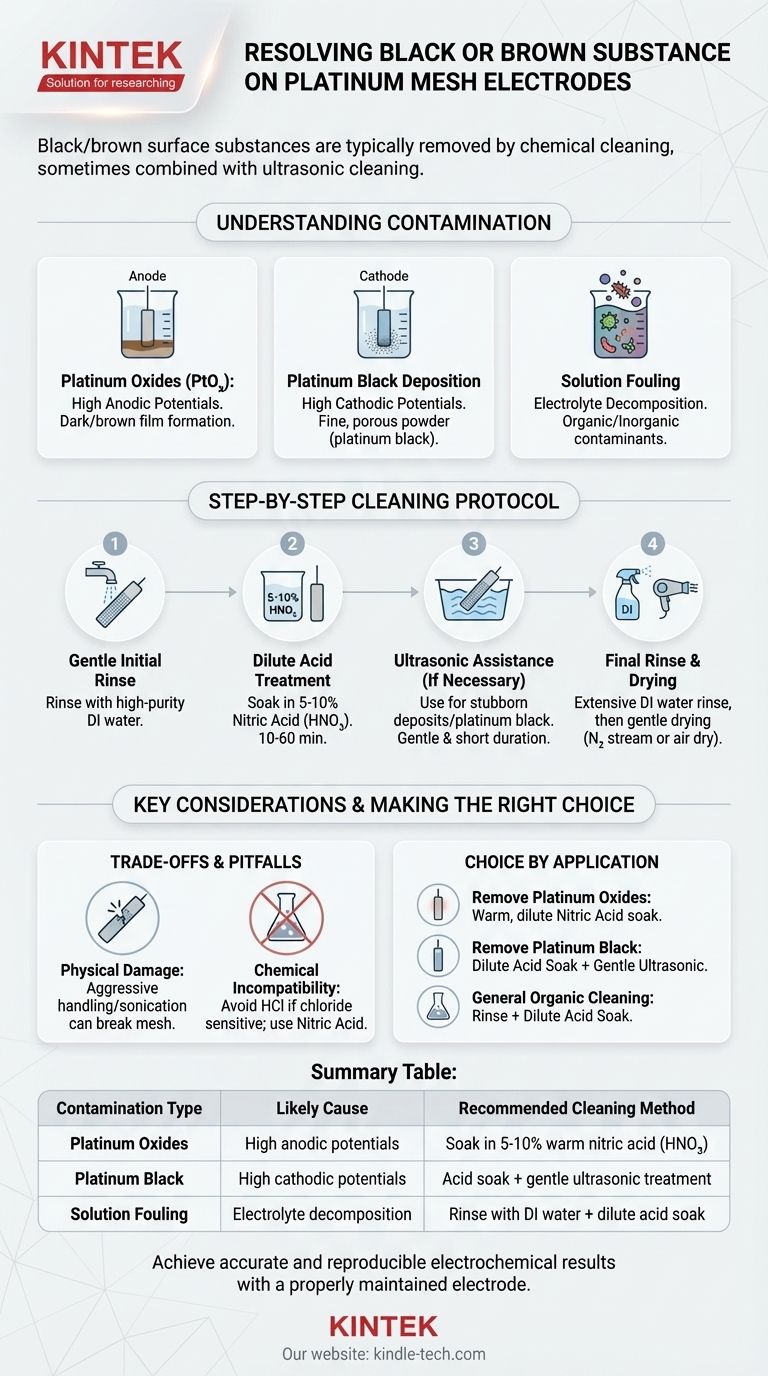
The appearance of a black or brown substance on your platinum electrode is a sign of surface contamination or modification that compromises its performance. The solution is not just to clean it, but to understand the likely cause to apply the correct cleaning protocol and prevent its recurrence.

What Is This Contamination?
Before cleaning, it's critical to understand what you're likely dealing with. The discoloration on a platinum electrode is rarely a simple stain; it's a chemical change to the surface.
Possibility 1: Platinum Oxides
Under certain electrochemical conditions, particularly high positive (anodic) potentials, a thin layer of platinum oxide (PtOₓ) can form. These oxides often appear as a dark or brown film on the otherwise bright metallic surface.
Possibility 2: Platinum Black Deposition
Conversely, under high negative (cathodic) potentials, platinum ions present in some solutions can be re-deposited onto the mesh as a very fine, porous powder. This high-surface-area material is known as platinum black and is a common cause of this issue.
Possibility 3: Solution Fouling
The substance may not be platinum at all. It could be the decomposition products of your electrolyte or the deposition of organic or inorganic contaminants from your solution. This is common in complex chemical mixtures or long-duration experiments.
A Step-by-Step Cleaning Protocol
A methodical approach ensures the electrode is cleaned effectively without causing damage. Always perform these steps in a well-ventilated area with appropriate personal protective equipment (PPE).
Step 1: Gentle Initial Rinse
Start by thoroughly rinsing the electrode with high-purity deionized (DI) water. This removes any loosely adhered salts or residual electrolyte from the mesh surface.
Step 2: Dilute Acid Treatment
Soak the electrode in a beaker containing a dilute acid solution. A 5-10% solution of nitric acid (HNO₃) is a common and effective choice for removing most oxides and organic residues. The soaking time can range from 10 minutes to an hour, depending on the severity.
Step 3: Ultrasonic Assistance (If Necessary)
If the acid soak alone is insufficient, place the beaker containing the electrode and dilute acid into an ultrasonic bath. The high-frequency vibrations will help dislodge stubborn particulate matter like platinum black from the fine mesh structure. Use this method judiciously, as prolonged sonication can physically damage the delicate mesh.
Step 4: Final Rinse and Drying
After cleaning, it is crucial to rinse the electrode extensively with DI water to remove every trace of the cleaning acid. You can then gently dry the electrode, often with a stream of nitrogen or by letting it air dry, before storing it properly.
Understanding the Trade-offs and Pitfalls
While cleaning is necessary, improper methods can create more problems than they solve.
Chemical Compatibility is Crucial
Be mindful of your future experiments. For example, if your work is sensitive to chloride ions, avoid using hydrochloric acid (HCl) for cleaning, as trace amounts may remain and interfere with results. Nitric acid is a more general-purpose choice.
The Risk of Over-Cleaning
Aggressive cleaning is not always better. Overly concentrated acids, excessively long soaking times, or frequent use of powerful reagents like aqua regia can slowly etch and dissolve the platinum itself. This alters the electrode's surface area and geometry, permanently changing its electrochemical behavior.
Physical Damage to the Mesh
A platinum mesh is a delicate structure. Aggressive handling, contact with hard surfaces, or prolonged, high-power sonication can easily bend, warp, or break the fine wires, rendering the electrode useless.
Making the Right Choice for Your Application
Your cleaning strategy should be guided by the type of contamination you suspect and the sensitivity of your work.
- If your primary focus is removing platinum oxides from anodic processes: A soak in warm, dilute nitric acid is typically the most effective and safest method.
- If your primary focus is removing deposited platinum black from cathodic processes: A combination of a dilute acid soak followed by a short, gentle ultrasonic treatment is often required to dislodge the fine particles.
- If your primary focus is general cleaning after use in organic solutions: A simple rinse followed by a soak in a suitable acid (like nitric) or even an electrochemical cleaning cycle may be sufficient.
Ultimately, maintaining the integrity of your platinum electrode's surface is essential for achieving accurate and reproducible results.
Summary Table:
| Contamination Type | Likely Cause | Recommended Cleaning Method |
|---|---|---|
| Platinum Oxides (PtOₓ) | High anodic potentials | Soak in 5-10% warm nitric acid (HNO₃) |
| Platinum Black | High cathodic potentials | Acid soak + gentle ultrasonic treatment |
| Solution Fouling | Electrolyte/organic decomposition | Rinse with DI water + dilute acid soak |
Achieve accurate and reproducible electrochemical results with a properly maintained electrode. The experts at KINTEK specialize in providing high-quality lab equipment and consumables, including platinum electrodes and the appropriate cleaning solutions. Our team can help you select the right tools and protocols to extend the life of your sensitive instrumentation. Contact our specialists today for a consultation tailored to your laboratory's specific needs.
Visual Guide
Related Products
- Platinum Auxiliary Electrode for Laboratory Use
- Custom PTFE Teflon Parts Manufacturer Corrosion Resistant Cleaning Rack Flower Basket
- Custom PTFE Teflon Parts Manufacturer for Conductive Glass Substrate Cleaning Rack
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- Custom PTFE Teflon Parts Manufacturer for Hollow Cleaning Basket and Rack Carrier
People Also Ask
- Why is a platinum electrode typically selected as the auxiliary or counter electrode? Unlock Precise Data Accuracy
- Why is platinum a good counter electrode? For Superior Chemical Inertness and Electron Transfer
- Why is a platinum wire (PtW) counter electrode preferred for cathode LSV tests? Ensure High-Precision Research
- Why is platinum typically selected as the auxiliary electrode for electrochemical testing of oxazoline inhibitors?
- What is the benefit of using a platinized platinum wire as a counter electrode? Optimize Operando Study Precision

















