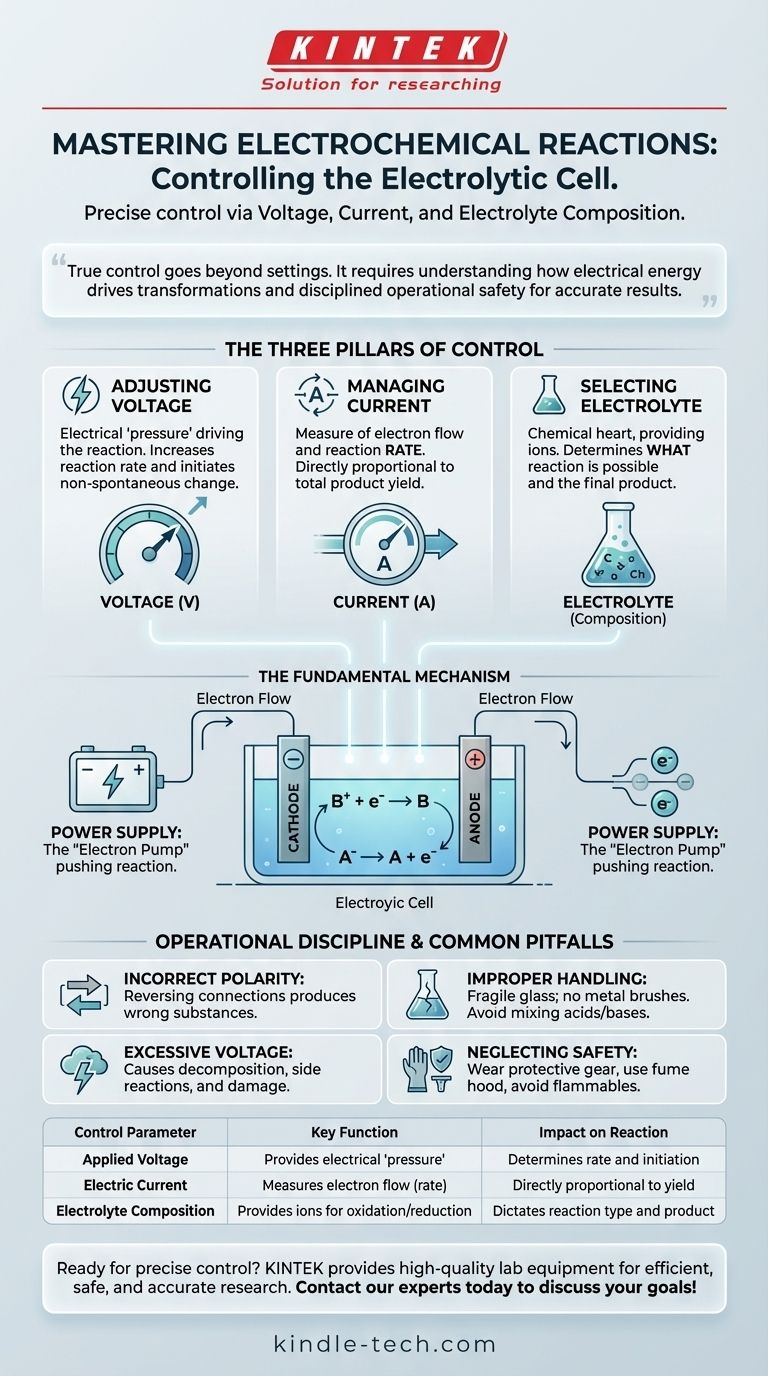
Controlling the reaction in an electrolytic cell is achieved by precisely manipulating three key parameters: the applied voltage, the resulting current, and the chemical composition of the electrolyte. These factors work together to dictate the rate, efficiency, and outcome of the chemical transformation you are trying to induce.
True control over an electrolytic cell goes beyond simply adjusting settings. It requires a fundamental understanding of how electrical energy drives specific chemical transformations and a disciplined approach to operational safety to ensure repeatable, accurate results.

The Three Pillars of Electrochemical Control
To master the cell's output, you must understand how each primary lever affects the reaction. These are not independent variables; a change in one will invariably impact the others.
H3: Adjusting Applied Voltage
Voltage is the electrical "pressure" or driving force behind the reaction. It provides the energy required to force a non-spontaneous chemical change.
Increasing the voltage generally increases the reaction rate, but only up to a point. It is the most direct way to initiate and sustain the process.
H3: Managing Electric Current
Current represents the flow of electrons through the circuit. It is a direct measure of the rate at which the reaction is occurring.
The amount of product formed over a period is directly proportional to the total charge (current x time) that has passed. Therefore, managing current is essential for controlling the yield.
H3: Selecting the Electrolyte Composition
The electrolyte is the chemical heart of the system, providing the ions that will be oxidized or reduced. The choice of electrolyte determines what reaction is possible.
Using a different electrolyte fundamentally changes the products you will create. Its purity and concentration are critical for preventing unwanted side reactions.
The Fundamental Mechanism: How Control Works
An electrolytic cell uses external energy to drive a reaction that would not happen on its own. Your control inputs directly manipulate this process at the atomic level.
H3: The Role of the Anode (Oxidation)
The anode is the positive electrode. The external power supply pulls electrons away from it, forcing a chemical species in the electrolyte to lose electrons, or become oxidized (e.g., A⁻ → A + e⁻).
H3: The Role of the Cathode (Reduction)
The cathode is the negative electrode. The power supply pushes an accumulation of electrons onto it. These electrons are then consumed by a chemical species in the electrolyte, which becomes reduced (e.g., B⁺ + e⁻ → B).
H3: The Power Supply's Function
The external power supply acts as an "electron pump." It creates the voltage potential that moves electrons from the anode to the cathode, forcing the oxidation and reduction reactions to occur and creating your desired product.
Common Pitfalls and Operational Discipline
Theoretical control is useless without rigorous operational practice. Mistakes can lead to failed experiments, damaged equipment, or serious safety hazards.
H3: Incorrect Electrode Polarity
Reversing the anode and cathode connections will reverse the intended reactions. This is a simple but critical error to avoid, as it will produce the wrong substances at each electrode.
H3: Excessive Voltage and Side Reactions
Applying too much voltage is a common mistake. It can cause the electrolyte itself (often water) to decompose or can damage the electrode surfaces. This reduces efficiency and contaminates your product.
H3: Improper Cell Handling and Cleaning
The glass cell body is fragile and must be handled with care. Never use metal brushes for cleaning, as scratches can weaken the glass. Critically, never mix acids and bases during cleaning, as this can cause a dangerous exothermic reaction.
H3: Neglecting Personal and Environmental Safety
Always wear protective gloves and glasses when handling corrosive electrolytes. Work in a well-ventilated fume hood to avoid inhaling harmful gases. Keep flammable materials and open flames far away from the apparatus to prevent fire or explosion.
Tailoring Control to Your Objective
Your control strategy depends entirely on your experimental goal. Use these principles to guide your approach.
- If your primary focus is reaction speed: Carefully increase the applied voltage while monitoring the current, being mindful of the threshold for unwanted side reactions.
- If your primary focus is product purity: Prioritize selecting a highly specific electrolyte and operate at the lowest effective voltage to minimize contamination from side reactions.
- If your primary focus is safety and repeatability: Establish a strict protocol for electrode connection, voltage limits, and personal protective equipment before beginning any experiment.
Mastering these principles transforms the electrolytic cell from a simple apparatus into a precise tool for chemical synthesis.
Summary Table:
| Control Parameter | Key Function | Impact on Reaction |
|---|---|---|
| Applied Voltage | Provides the electrical "pressure" to drive the reaction. | Determines the reaction rate and initiation. |
| Electric Current | Measures the flow of electrons (reaction rate). | Directly proportional to the product yield. |
| Electrolyte Composition | Provides the ions for oxidation/reduction. | Dictates what chemical reaction is possible and the final product. |
Ready to achieve precise control over your electrochemical processes? KINTEK specializes in high-quality lab equipment and consumables for all your laboratory needs. Whether you're setting up a new electrolysis experiment or optimizing an existing one, our expertise and reliable products ensure your work is efficient, safe, and accurate. Contact our experts today to discuss how we can support your research and development goals!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What are the key features of the five-port water bath electrolytic cell? Precision Control for Electrochemical Experiments
- What are the standard aperture specifications of the electrolytic cell? Key Sizes for Your Electrochemical Setup
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What are the procedures for after using a double-layer water-bath electrolytic cell? Ensure Equipment Longevity and Data Accuracy
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments



















