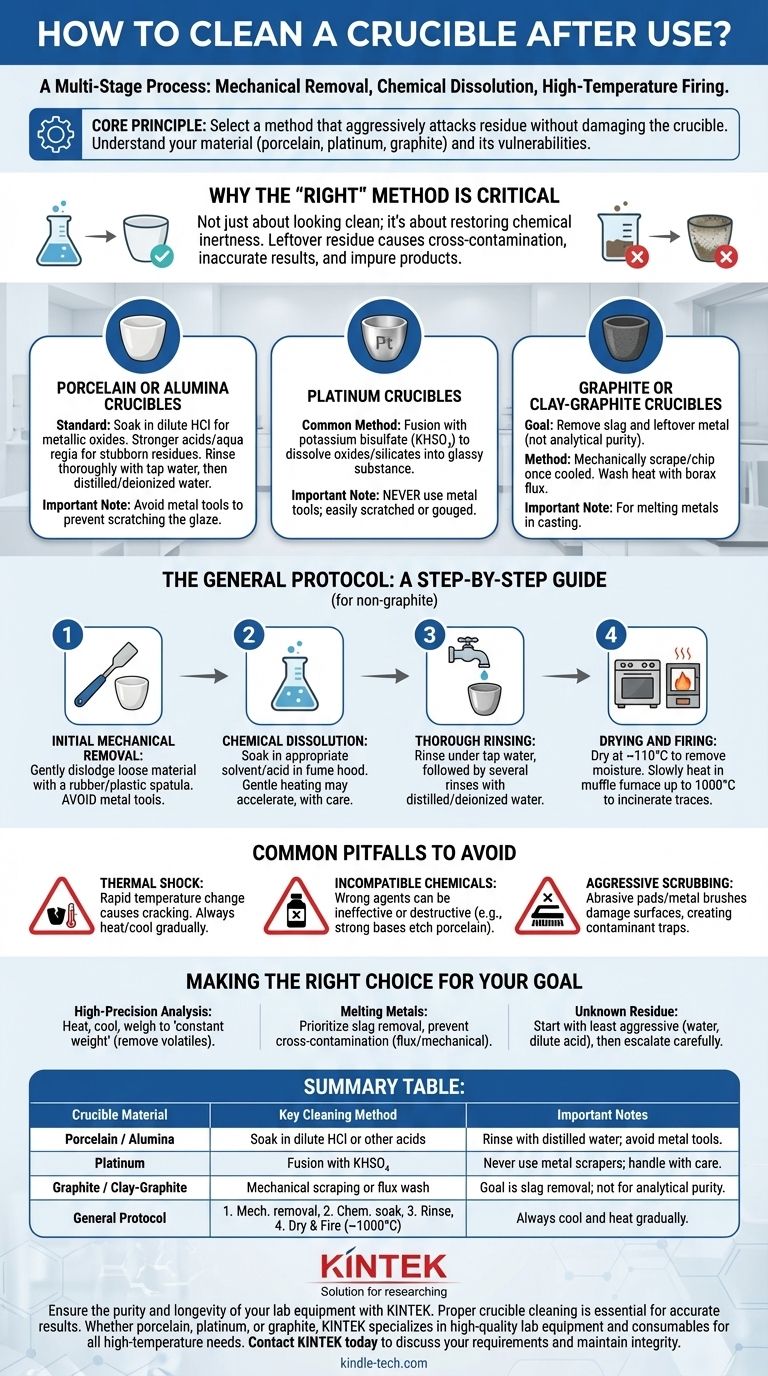
To clean a crucible, you must follow a multi-stage process that includes mechanical removal of bulk residue, chemical dissolution of remaining contaminants, and a final high-temperature firing to burn off any trace impurities. The specific chemical used and the final temperature depend entirely on the crucible's material and the nature of the substance it held.
The core principle of crucible cleaning is not to follow a single recipe, but to select a method that aggressively attacks the residue without damaging the crucible itself. This requires a fundamental understanding of your crucible's material—porcelain, platinum, or graphite—and its chemical vulnerabilities.

Why the "Right" Method Is Critical
Cleaning a crucible is not just about making it look clean; it's about restoring it to a chemically inert state. Leftover residue from a previous process can contaminate your next sample, leading to inaccurate analytical results or impure final products in metallurgy.
For Porcelain or Alumina Crucibles
These are the most common types found in analytical chemistry labs. They are chemically resistant but brittle.
The standard procedure involves soaking the crucible in a solution that dissolves the specific residue. For example, a dilute solution of hydrochloric acid (HCl) is effective for dissolving many metallic oxides. For more stubborn inorganic residues, stronger acids or even aqua regia may be required under strict safety protocols.
After chemical treatment, the crucible must be rinsed thoroughly, first with tap water and finally with distilled or deionized water to remove any mineral contaminants.
For Platinum Crucibles
Platinum is extremely expensive and requires careful handling. While resistant to most acids, it can be damaged by certain processes.
A common method for cleaning inorganic material from platinum is to perform a fusion with potassium bisulfate (KHSO₄). The salt is melted inside the crucible, dissolving metallic oxides and silicates into a glassy substance that can be poured out and dissolved in water.
Never use metal tools to scrape a platinum crucible, as this can easily scratch or gouge the soft metal.
For Graphite or Clay-Graphite Crucibles
These are used for melting metals in casting and foundry work. Their cleaning process is fundamentally different.
The goal is not to achieve analytical purity but to remove slag and leftover metal. This is typically done by mechanically scraping or chipping out the residue once the crucible has completely cooled.
For a more thorough clean, a "wash heat" can be performed by melting a flux like borax in the crucible. The molten borax dissolves many metallic oxides, which can then be poured out, leaving a cleaner interior surface.
The General Protocol: A Step-by-Step Guide
While specifics vary, a safe, general-purpose workflow provides a reliable starting point for most laboratory (non-graphite) crucibles.
Step 1: Initial Mechanical Removal
Once the crucible is cool, use a rubber policeman or a plastic spatula to gently dislodge and remove any loose, non-adhered material. Avoid metal tools that can scratch the interior glaze of a porcelain crucible.
Step 2: Chemical Dissolution
Submerge the crucible in an appropriate solvent or acid within a fume hood. Allow it to soak until the residue is visibly dissolved or loosened. Gentle heating can accelerate this process, but must be done with extreme care to avoid splashing corrosive chemicals.
Step 3: Thorough Rinsing
After the chemical soak, rinse the crucible under running tap water to remove the majority of the cleaning agent. Follow this with several rinses using distilled or deionized water to eliminate any final ionic contaminants.
Step 4: Drying and Firing
Place the rinsed crucible in a drying oven set to approximately 110°C for several hours to remove all moisture. This prevents cracking from thermal shock in the next step.
Finally, place the dry crucible in a muffle furnace and slowly heat it to a high temperature, often up to 1000°C. This final firing incinerates any remaining organic traces and ensures the crucible is in a stable, inert state for its next use.
Common Pitfalls to Avoid
Understanding what can go wrong is as important as knowing the procedure itself.
The Danger of Thermal Shock
Never place a hot crucible on a cold surface or a cold crucible into a pre-heated furnace. The rapid temperature change will induce stress and cause cracking. Always allow for gradual heating and cooling.
Incompatible Chemical Agents
Using the wrong chemical can be ineffective at best and destructive at worst. For example, strong bases (like sodium hydroxide) should not be used to clean porcelain crucibles for extended periods, as they can slowly etch the glazed surface.
Aggressive Physical Scrubbing
Scouring a crucible with abrasive pads or metal brushes will damage its interior surface. These micro-scratches create sites where future contaminants can get trapped, making the crucible progressively harder to clean and less reliable.
Making the Right Choice for Your Goal
Your specific objective dictates the required level of cleanliness and the appropriate method.
- If your primary focus is high-precision gravimetric analysis: You must heat, cool, and weigh the crucible repeatedly until you achieve a "constant weight," ensuring all volatile substances are gone.
- If your primary focus is melting metals for casting: Your priority is removing slag and preventing cross-contamination between different alloys using flux and mechanical cleaning.
- If your primary focus is removing an unknown residue: Start with the least aggressive methods—water, then a dilute acid—before escalating to stronger chemicals, always respecting the limitations of your crucible's material.
Ultimately, a meticulously cleaned and prepared crucible is the foundation of any successful high-temperature work, ensuring the integrity and purity of your results.
Summary Table:
| Crucible Material | Key Cleaning Method | Important Notes |
|---|---|---|
| Porcelain / Alumina | Soak in dilute HCl or other acids | Rinse with distilled water; avoid metal tools to prevent scratching. |
| Platinum | Fusion with potassium bisulfate (KHSO₄) | Never use metal scrapers; handle with extreme care due to high cost. |
| Graphite / Clay-Graphite | Mechanical scraping or flux (e.g., borax) wash | Goal is slag removal; not for analytical purity. |
| General Protocol | 1. Mechanical removal 2. Chemical soak 3. Rinsing 4. Drying & Firing (~1000°C) | Always cool and heat gradually to avoid thermal shock. |
Ensure the purity and longevity of your lab equipment with KINTEK. Proper crucible cleaning is essential for accurate analytical results and efficient metal melting processes. Whether you work with porcelain, platinum, or graphite crucibles, having the right tools and consumables is key. KINTEK specializes in high-quality lab equipment and consumables, serving laboratories with reliable solutions for all their high-temperature needs.
Contact KINTEK today to discuss your specific requirements and let our experts help you maintain the integrity of your work.
Visual Guide
Related Products
- High Purity Pure Graphite Crucible for Evaporation
- High Purity Pure Graphite Crucible for Electron Beam Evaporation
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
People Also Ask
- Can you melt copper in a ceramic crucible? Yes, with the right crucible choice.
- Why are alumina crucibles preferred for DSC analysis of SiCp/2009Al? Ensure High-Temperature Chemical Inertness
- What are the advantages of using alumina (corundum) crucibles in vitrification? Ensure Pure, High-Temp Results
- What crucibles are used in muffle furnace? Choose the Right Material for Your High-Temp Application
- How much heat can a ceramic crucible withstand? A Guide to Material-Specific Temperature Limits
- What material is used for induction furnace crucibles? Match Your Metal & Frequency for Optimal Melting
- What are the advantages of using a Glassy Carbon Crucible for fluoride salts? Ensure Purity up to 1000°C
- Why are alumina crucibles selected for FeCrAl coating experiments? Ensure Data Fidelity at High Temperatures



















