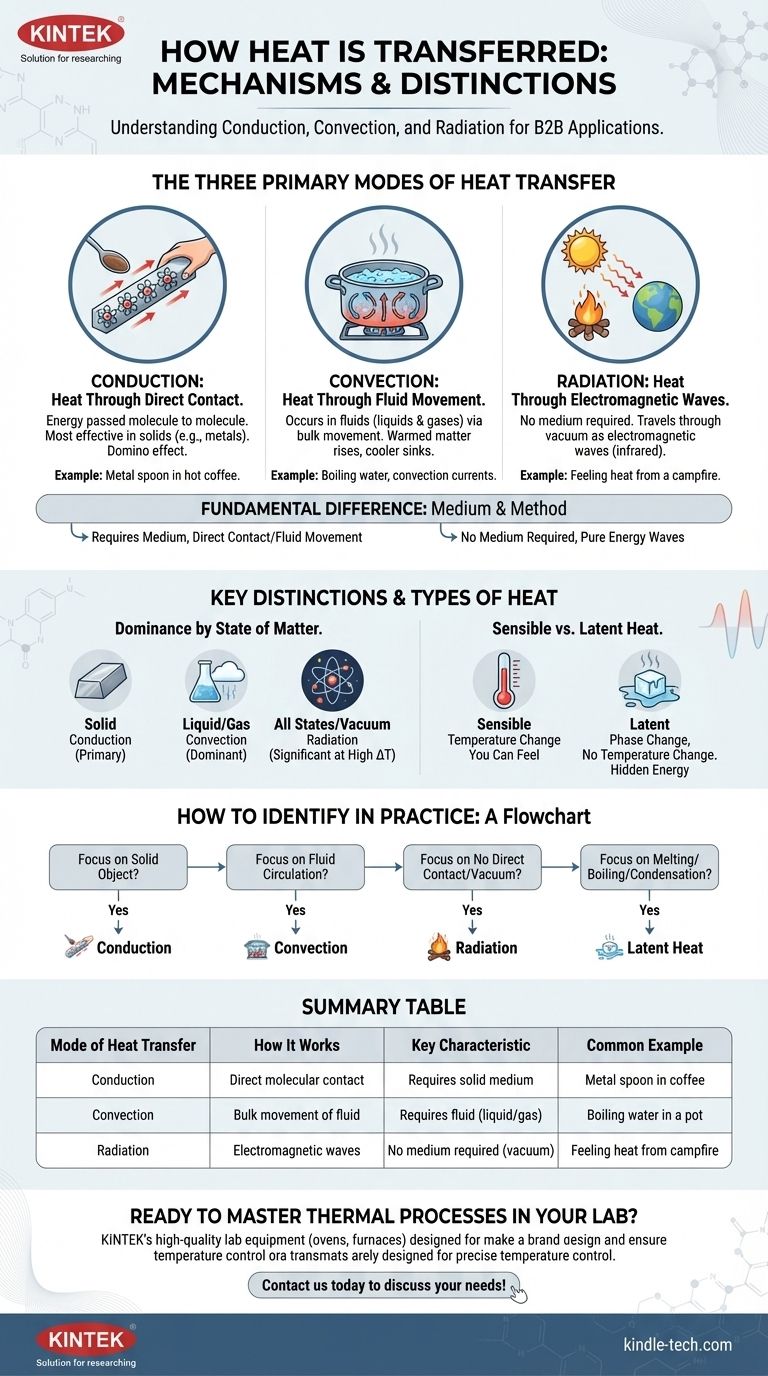
At its core, heat is transferred through three distinct physical mechanisms: conduction, convection, and radiation. Conduction moves heat through direct molecular contact, convection moves heat via the bulk flow of fluids, and radiation transfers heat as electromagnetic waves through any medium, including the vacuum of space.
The fundamental difference lies in the medium and method: conduction requires direct contact, convection requires fluid movement, and radiation requires no medium at all, traveling as pure energy.

The Three Primary Modes of Heat Transfer
To understand how thermal energy moves from a hotter area to a colder one, we must break down each of the three primary modes. They often occur simultaneously, but one is typically dominant depending on the situation.
Conduction: Heat Through Direct Contact
Conduction is the transfer of heat between substances that are in direct contact with each other. The energy is passed from one vibrating molecule to the next without any of the molecules changing their position.
Think of it as a line of dominoes. The first domino falls and transfers its energy to the next, which then transfers it to the next, and so on. The dominoes themselves don't travel down the line, only the energy does.
This mode is most effective in solids, especially metals, where atoms are packed closely together. A classic example is a metal spoon heating up when placed in a hot cup of coffee.
Convection: Heat Through Fluid Movement
Convection is the transfer of heat by the actual movement of warmed matter. This process only occurs in fluids—liquids and gases—where molecules are free to move around.
When a fluid is heated, it expands, becomes less dense, and rises. Cooler, denser fluid then sinks to take its place, gets heated, and rises in turn. This continuous circulation is called a convection current.
Boiling water is a perfect example. Heat from the stove's element is conducted to the bottom of the pot, which then heats the water at the bottom. This hot water rises, and cooler water from the top sinks to be heated, creating a rolling boil.
Radiation: Heat Through Electromagnetic Waves
Radiation is heat transfer that does not rely on any contact between the heat source and the heated object. It operates by emitting energy in the form of electromagnetic waves, primarily in the infrared spectrum.
Unlike conduction and convection, radiation can travel through the emptiness of space. This is how the sun's energy travels 93 million miles to warm the Earth.
You can feel this mode of transfer when you stand near a campfire. The heat you feel on your face is not from conduction (you aren't touching the fire) or convection (the hot air is rising away from you), but from thermal radiation.
Understanding the Key Distinctions
Each mode of heat transfer has unique characteristics that determine where and how it operates. Understanding these distinctions is key to analyzing any thermal system.
The Role of a Medium
The most fundamental difference is the need for a medium. Conduction and convection absolutely require a medium—solid for conduction, fluid for convection—to transfer energy.
Radiation, however, needs no medium. It is the only form of heat transfer that can occur in a perfect vacuum.
Dominance by State of Matter
The state of matter heavily influences which mode is most effective. Conduction is the primary mode of heat transfer within solids. Convection is the dominant mode within liquids and gases.
Radiation occurs across all states of matter and is significant at high temperature differences, regardless of the medium.
A Different Dimension: Sensible vs. Latent Heat
Separate from the modes of transfer is the type of heat being transferred. This is categorized as either sensible or latent heat.
Sensible Heat: The Temperature You Can Feel
Sensible heat is the energy transferred that results in a change of temperature in an object. It's "sensible" because you can measure it with a thermometer.
When you heat a pot of water from 20°C to 80°C, the energy added is sensible heat.
Latent Heat: The Hidden Energy of Phase Change
Latent heat is the energy absorbed or released when a substance changes its physical state (a phase change), such as from solid to liquid or liquid to gas, without changing its temperature.
For example, when ice at 0°C melts into water at 0°C, it must absorb a significant amount of latent heat. This "hidden" energy is used to break the molecular bonds of the ice structure, not to raise the temperature.
How to Identify Heat Transfer in Practice
By understanding these principles, you can easily identify the dominant form of heat transfer in any scenario.
- If your focus is on heat moving through a solid object: You are primarily dealing with conduction.
- If your focus is on heat circulating in air or water: You are primarily dealing with convection.
- If your focus is on heat traveling from a source with no direct contact: You are primarily dealing with radiation.
- If your focus is on melting, freezing, boiling, or condensation: The critical energy involved is latent heat.
Understanding these mechanisms empowers you to analyze how energy moves through the world, from a simple cup of coffee to the engine in a car.
Summary Table:
| Mode of Heat Transfer | How It Works | Key Characteristic | Common Example |
|---|---|---|---|
| Conduction | Direct molecular contact | Requires a solid medium | Metal spoon in hot coffee |
| Convection | Bulk movement of fluid | Requires a fluid (liquid/gas) | Boiling water in a pot |
| Radiation | Electromagnetic waves | No medium required (works in vacuum) | Feeling heat from a campfire |
Ready to Master Thermal Processes in Your Lab?
Understanding heat transfer is fundamental to countless laboratory processes, from sample preparation to material synthesis. The right equipment ensures precise temperature control and efficient energy use.
KINTEK specializes in high-quality lab equipment, including ovens, furnaces, and heating mantles, designed with these thermal principles in mind to deliver reliable, consistent, and safe performance for your specific applications.
Let our experts help you select the perfect heating solution for your needs. Contact us today to discuss how we can support your laboratory's thermal processing challenges!
Visual Guide
Related Products
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
People Also Ask
- What is the purpose of a hot pressing system following the reduction of iron powder in a fluidized bed? Stabilize DRI
- What is the function of a hydraulic heat press? Perfecting Solid-State Battery Polymer Membranes
- What is the purpose of using a laboratory hydraulic press for nanocomposites? Ensure Precise Material Characterization
- What role does a laboratory hydraulic hot press play in rice husk-based composite boards? Achieve Structural Density
- Why is precise pressure control via a hydraulic system necessary during hot pressing? Optimize Nanocopper Performance



















