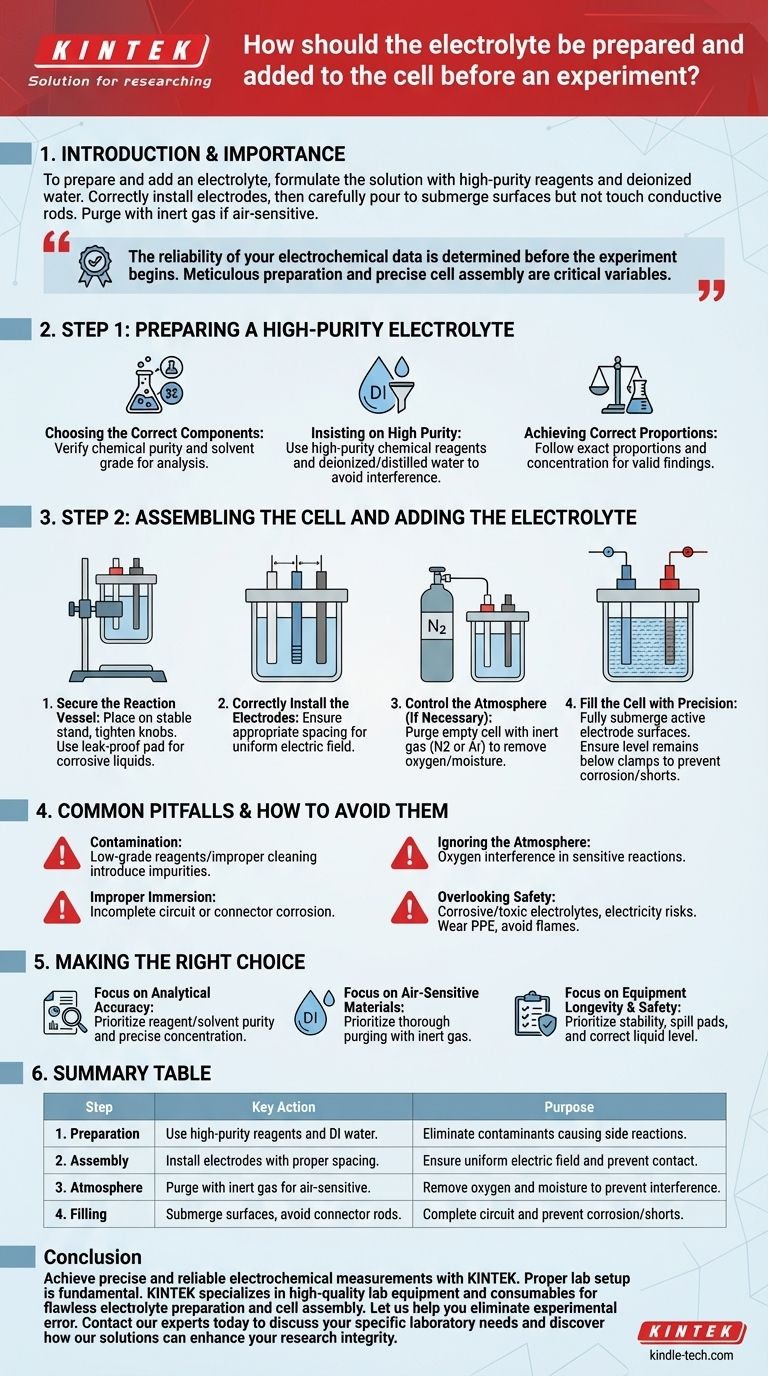
To prepare and add an electrolyte, you must first formulate the solution with high-purity reagents and deionized water according to your experimental requirements. After correctly installing the electrodes in the cell, carefully pour in the electrolyte to a level that fully submerges the electrode surfaces but does not touch the conductive connecting rods. If your experiment is air-sensitive, you must purge the cell with an inert gas like nitrogen before adding the liquid.
The reliability of your electrochemical data is determined before the experiment ever begins. Meticulous preparation of the electrolyte and precise cell assembly are not preliminary chores—they are the most critical variables you control.
Step 1: Preparing a High-Purity Electrolyte
The quality of your electrolyte directly dictates the quality of your results. Any impurity can introduce unintended side reactions, fundamentally compromising your data.
Choosing the Correct Components
Based on your specific experimental goals, you must select a suitable electrolyte. Verify that the chemical's purity and the solvent's grade meet the standards required for your analysis.
Insisting on High Purity
Always use high-purity chemical reagents and deionized or distilled water. Common tap water contains ions and organic matter that will interfere with your experiment, producing misleading or non-repeatable results.
Achieving Correct Proportions
Prepare the solution according to the exact proportions and concentration specified by your experimental protocol. Inaccurate concentrations will shift reaction potentials and alter electrode kinetics, invalidating your findings.
Step 2: Assembling the Cell and Adding the Electrolyte
Proper assembly ensures a stable, safe, and correctly configured electrochemical system. Each step is designed to eliminate common sources of experimental error.
Secure the Reaction Vessel
First, place your electrolytic cell onto a stable stand and tighten any fixing knobs. This ensures the cell remains vertical and does not wobble. For corrosive electrolytes, place a leak-proof pad underneath the cell to protect your work surface.
Correctly Install the Electrodes
Install the working, reference, and counter electrodes in the reaction vessel. Ensure there is appropriate spacing between them to facilitate a uniform electric field and prevent contact.
Control the Atmosphere (If Necessary)
For air-sensitive experiments, you must control the environment. Before adding the electrolyte, purge the empty cell with an inert gas, such as high-purity nitrogen or argon, to displace all residual oxygen and moisture.
Fill the Cell with Precision
Pour the prepared electrolyte into the cell. The goal is to fully submerge the active surfaces of all three electrodes. Crucially, you must also ensure the liquid level remains below the clamps or wires connecting to the electrode rods to prevent corrosion and short-circuiting.
Common Pitfalls and How to Avoid Them
Even minor oversights during setup can have an outsized impact on your experiment's outcome. Understanding these risks is key to generating trustworthy data.
The Pitfall of Contamination
Using low-grade reagents or failing to properly clean glassware introduces contaminants. These impurities can act as catalysts, inhibitors, or competing reactants, making it impossible to study your system of interest in isolation.
The Pitfall of Improper Immersion
If the electrode surfaces are not fully submerged, you will have an incomplete circuit and inaccurate current density measurements. Conversely, if the electrolyte touches the alligator clips or connecting rods, you will introduce unwanted metal corrosion and parasitic reactions.
The Pitfall of Ignoring the Atmosphere
Many electrochemical reactions are highly sensitive to oxygen. Failing to de-aerate the electrolyte for such systems will lead to oxygen reduction becoming a dominant, and unwanted, side reaction at your working electrode.
The Pitfall of Overlooking Safety
Electrolytes can be corrosive, toxic, or flammable, and the experiment involves electricity. Always wear appropriate personal protective equipment (PPE). Avoid direct contact with electrodes and electrolyte, and never have open flames or spark sources near a cell that may produce flammable gases like hydrogen.
Making the Right Choice for Your Experiment
Your experimental goals dictate which procedural details demand the most attention.
- If your primary focus is analytical accuracy: Your highest priority is the purity of your reagents and solvent, and the precise concentration of your final electrolyte solution.
- If your primary focus is studying air-sensitive materials: The most critical step is thoroughly purging the cell and electrolyte with an inert gas to remove all traces of oxygen.
- If your primary focus is equipment longevity and safety: Always ensure the cell is stable, use a spill pad for corrosive liquids, and verify the electrolyte level never touches the electrode connectors.
Ultimately, procedural discipline is the foundation of reliable electrochemical data.

Summary Table:
| Step | Key Action | Purpose |
|---|---|---|
| 1. Preparation | Use high-purity reagents and deionized water. | Eliminate contaminants that cause side reactions. |
| 2. Assembly | Install electrodes with proper spacing. | Ensure a uniform electric field and prevent contact. |
| 3. Atmosphere | Purge cell with inert gas for air-sensitive experiments. | Remove oxygen and moisture to prevent interference. |
| 4. Filling | Submerge electrode surfaces, avoid connector rods. | Complete the circuit and prevent corrosion/shorts. |
Achieve precise and reliable electrochemical measurements with KINTEK.
Proper lab setup is fundamental to your success. KINTEK specializes in high-quality lab equipment and consumables, providing the reliable tools you need—from pure reagents to stable electrochemical cells—to ensure your electrolyte preparation and cell assembly are flawless.
Let us help you eliminate experimental error. Contact our experts today to discuss your specific laboratory needs and discover how our solutions can enhance your research integrity.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
People Also Ask
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- How should the five-port water bath electrolytic cell be operated during an experiment? Master Precise Control for Reliable Results
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol



















