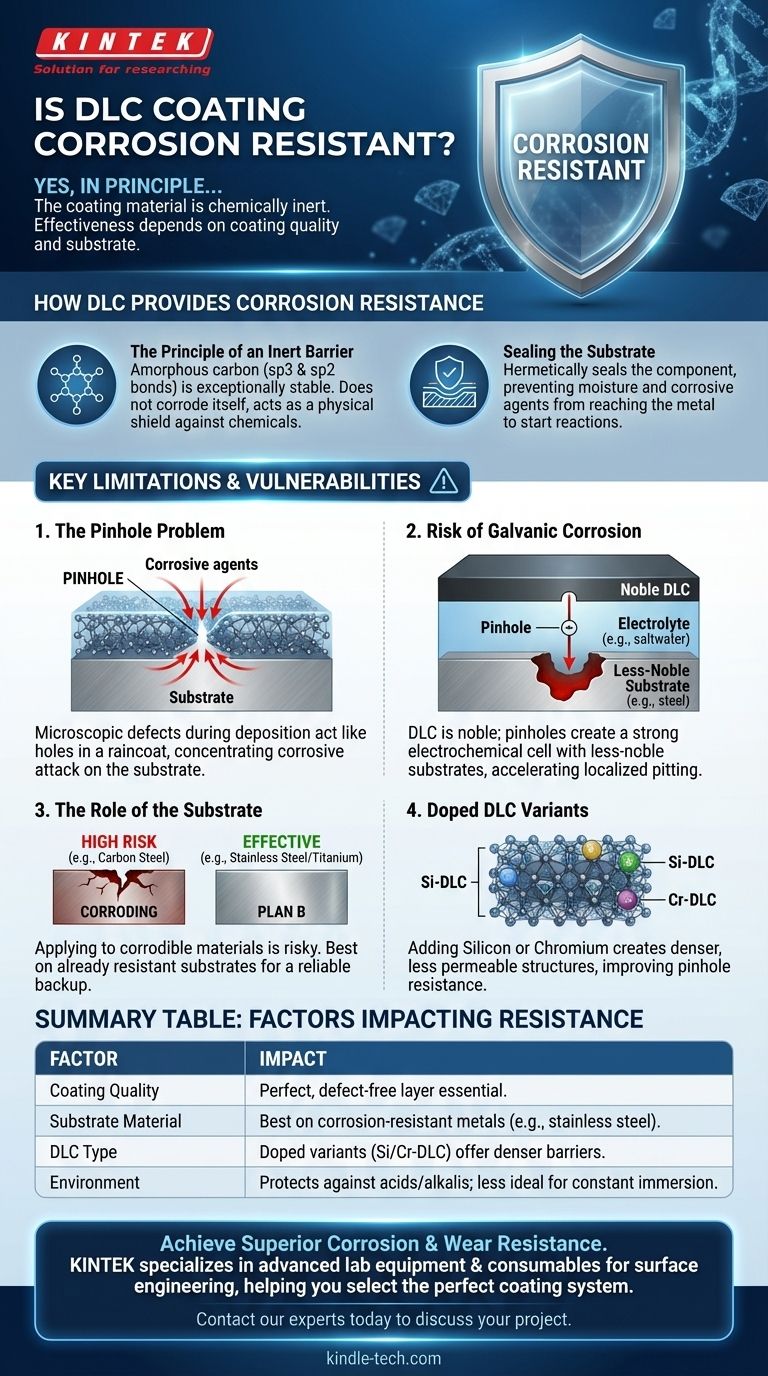
In principle, yes. Diamond-Like Carbon (DLC) coating is highly corrosion-resistant because the material itself is chemically inert and does not react with acids, alkalis, or other corrosive agents. However, its effectiveness in a real-world application depends entirely on the quality of the coating and the substrate it is applied to.
The corrosion resistance of a DLC-coated part is not determined by the DLC material itself, but by the coating's ability to form a perfect, impenetrable barrier. Any microscopic pinhole or defect can lead to localized corrosion of the underlying material.

How DLC Provides Corrosion Resistance
DLC's protective mechanism is straightforward: it acts as a physical barrier. It isolates the underlying component, or substrate, from the corrosive environment.
The Principle of an Inert Barrier
DLC is a form of amorphous carbon with a structure containing both diamond-like (sp3) and graphite-like (sp2) bonds. This structure makes it exceptionally stable and non-reactive, similar to a ceramic or a noble metal.
It doesn't corrode itself; its only job is to prevent moisture and corrosive chemicals from ever reaching the metal underneath.
Sealing the Substrate
Think of DLC as a high-performance paint. When applied perfectly, it hermetically seals the substrate. This barrier prevents the electrochemical reactions that cause rust and other forms of corrosion from ever starting.
Key Limitations of DLC for Corrosion Protection
Trusting DLC blindly is a common pitfall. The coating itself is inert, but the coating system (the combination of the DLC layer and the substrate) has critical vulnerabilities.
The Pinhole Problem
During the PVD or PACVD deposition process, microscopic defects known as pinholes can form. These are tiny, often invisible-to-the-eye voids that penetrate the full depth of the coating.
A single pinhole acts like a hole in a raincoat. The area under the hole gets wet, and in this case, the substrate is exposed. All corrosive attack will be concentrated at that tiny point.
Risk of Galvanic Corrosion
This situation is made worse by a phenomenon called galvanic corrosion. Because DLC is very noble (non-reactive), it creates a strong electrochemical cell with a less-noble substrate like steel when an electrolyte (like saltwater) is present.
This cell dramatically accelerates corrosion at the base of the pinhole, leading to rapid, localized pitting that can be more destructive than if the part were uncoated.
The Role of the Substrate
Applying DLC to a material that is already prone to corrosion, like plain carbon steel, is high-risk. Meticulous surface preparation is required to ensure a defect-free coating.
Conversely, applying DLC to an already corrosion-resistant material, such as 316 stainless steel or titanium, is a highly effective strategy. Here, the DLC adds superior wear and friction properties while the substrate provides a reliable "plan B" against corrosion if the coating is ever compromised.
Doped DLC Variants
Standard DLC (a-C:H) provides good general performance. However, for enhanced corrosion protection, specialized variants are often used. Adding elements like Silicon (Si-DLC) or Chromium (Cr-DLC) can create a denser, less permeable coating structure that is more resistant to pinhole formation.
Making the Right Choice for Your Application
To successfully use DLC for corrosion control, you must match the coating system to your specific goal and environment.
- If your primary focus is adding wear resistance to an already inert material (like stainless steel or titanium): DLC is an outstanding choice that complements the substrate's properties without creating new risks.
- If your primary focus is protecting a corrodible base metal (like tool steel): You must prioritize a thick, multi-layered, and potentially "doped" DLC, applied over a flawless surface finish.
- If the part will be in constant immersion or a highly aggressive chemical environment: A standard DLC is likely insufficient. Specify a dense, pinhole-resistant variant and consider a corrosion-resistant underlayer.
Ultimately, viewing DLC not as a simple coating but as an engineered surface system is the key to achieving reliable corrosion protection.
Summary Table:
| Factor | Impact on Corrosion Resistance |
|---|---|
| Coating Quality | A perfect, defect-free (pinhole-free) layer is essential. |
| Substrate Material | Best results on already corrosion-resistant metals (e.g., stainless steel). |
| DLC Type | Doped variants (e.g., Si-DLC, Cr-DLC) offer denser, more protective barriers. |
| Environment | Protects against acids, alkalis, and moisture; less ideal for constant immersion. |
Achieve superior corrosion and wear resistance for your components. The effectiveness of a DLC coating depends on expert application and engineering. KINTEK specializes in advanced lab equipment and consumables for surface engineering, helping you select and apply the perfect coating system for your specific needs. Don't leave your components' performance to chance—contact our experts today to discuss your project and ensure lasting protection.
Visual Guide
Related Products
- Custom CVD Diamond Coating for Lab Applications
- Electrolytic Electrochemical Cell for Coating Evaluation
- CVD Diamond Cutting Tool Blanks for Precision Machining
- Custom PTFE Teflon Parts Manufacturer Corrosion Resistant Cleaning Rack Flower Basket
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
People Also Ask
- What are the three types of coating? A Guide to Architectural, Industrial, and Special Purpose
- How long does diamond coating last? Maximize Lifespan with the Right Coating for Your Application
- Is diamond coating permanent? The Truth About Its Long-Lasting Durability
- What are diamond coated films? Enhance Materials with Super-Hard, Transparent Layers
- How are tools coated with diamond? Achieve Superior Hardness and Low Friction for Your Tools











